Paravalvular leak (PVL) is an uncommon yet serious complications associated with the implantation of surgical prosthetic valves and more recently recognized with transcatheter valves. Renewed interest has developed as relationships have emerged between the degree of paravalvular regurgitation and mortality.1 Percutaneous closure of PVLs has shown significant promise with contemporary success rates as high as 86%, especially in patients at high risk for surgical repair or replacement.2,3 Growth in the field of structural heart disease has afforded the adaptation of many technical and procedural advances in an attempt to further improve transcatheter success, while reducing the risk of complications. Recent publications in Revista Española de Cardiología by Cruz-González et al4 and Sánchez-Recalde et al5 exemplify the continued desire of interventionalists to innovate. It is their application of newer technologies and techniques that is allowing for expansion. For percutaneous PVL closure, the question remains, where do we go from here? We believe that a 3-fold approach, which focuses on better devices to fit the unique shapes of PVLs, more favorable sites of access, and improved imaging guidance, will further push the limits of this encouraging therapy.
PVLs are the result of an incomplete seal between the sewing ring and annulus for surgical prostheses or stent frame and aortic annulus/calcified leaflets for transcatheter aortic valves. For the former, this arises from abnormal pressure or traction forces after surgery, usually related to annular calcification, infection, suturing technique, and prosthetic size and shape. For the latter, PVL is typically a result of incomplete prosthesis apposition to the native aortic annulus due to calcification, annular eccentricity, valve undersizing, and/or malpositioning, irrespective of the prosthesis type: self- or balloon-expandable.6 Most of our experience in transcatheter closure and imaging revolves around PVLs in the setting of surgical prostheses. Despite the potential etiologies, the shape and track of each PVL varies. It is rare to find round PVL, with most having an oval or crescentic appearance. In addition, their tracks are rarely parallel and often run perpendicular with a serpiginous course. The physical characteristics of the surrounding tissue are equally difficult to determine and their response to closure devices nearly impossible to predict. Advances in 3-dimensional (3D) transesophageal echocardiography and 3D/4D computed tomographic angiography have greatly improved our evaluation and planning prior to percutaneous closure.
Initial strategies for percutaneous closure included umbrella devices and coils. These were eventually replaced with devices designed for closure of other cardiovascular defects (off-label) such as the Amplatzer family of devices (St Jude Medical, St Paul, Minnesota, United States): atrial and muscular ventricular septal occluders and ductal occluder. These devices are round or conical, filled with polyester fiber, and have either 1 or 2 retention discs. Their nitinol mesh design is less densely woven with a more rigid structure than more contemporary plugs, which make them less amenable to conformation. Currently, the Amplatzer vascular plug (AVP) II is the most commonly used device in the United States to close PVLs in surgical prostheses. It is constructed with a more densely layered nitinol mesh with 3 segments: a central lobe and 2 discs on either side.7 The AVP II is round in shape; however, the fine mesh does allow for some shape conformation.
Cruz-González et al4 and Sánchez-Recalde et al5 have revealed their experience with a unique vascular plug, the AVP III, with success rates as high as 94%. This device is made from the same nitinol mesh as the AVP II but has an oblong shape with 2 extended rims.7 It has a long axis diameter ranging from 4 to 14mm and a short axis diameter of 2 to 5mm. It can be delivered through 4 to 7 Fr sheaths or 6 to 9 Fr guide catheters. It is a niche device that can be used for whatever anatomy fits with its particular structure. However, clinical reports in the literature have been limited to the treatment of PVLs.8,9 The device is not widely available for use; it has European Commission approval to embolize blood vessels in the peripheral vasculature, but has not received Food and Drug Administration approval in the United States.
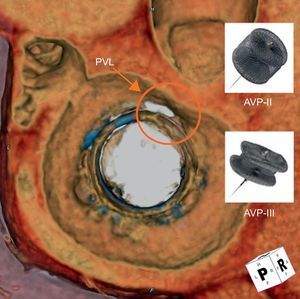
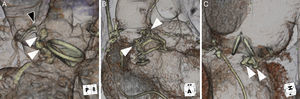
The choice of size for the AVP III is similar to the most commonly used sizes of the AVP II, typically 8 to 12mm. It is postulated that crescentic or oblong PVLs, the majority, may be more suitable for closure using the AVP III (Figure 1). With the AVP II, the middle lobe is molded into the PVL shape. For many large PVLs, this may require multiple devices deployed either sequentially or simultaneously; dense filling of the PVL with multiple devices may ensure proper sealing. In addition, the concept of multiple smaller devices rather than 1 or 2 larger devices has been suggested to have better sealing within the PVL and less interference with other cardiac structures.10 When oversizing AVP IIs, the center lobe is typically compressed by the PVL/surgical prosthesis, elongating the device and increasing the chances for device overhanging. Overhanging can potentially lead to mechanical obstruction of the coronary ostia (in the aortic position) or valvular flow, or cause prosthetic dysfunction, particularly for mechanical types (Figure 2). Multiple AVP IIIs may allow for even denser PVL channel filling of leaks, without these potential risks.
Three-dimensional reconstruction of cardiac computed tomographic angiography reveals a mitral bioprosthetic valve (en face view from the atrial side) with a crescentic shaped paravalvular leak (red circle) at the 11 to 12 o’clock location. The Amplatzer vascular plug III (oblong) may be more suitable for closure as it better conforms to the shape of the PVL, compared with the Amplatzer vascular plug II (round). AVP, Amplatzer vascular plug; PVL, paravalvular leak.
With oversizing of Amplatzer vascular plug IIs, the center lobe can be compressed by the paravalvular leak and surgical prosthesis. Elongation of the Amplatzer vascular plug II (white arrowheads) can lead to mechanical obstruction and risk of complications. A: Coronary occlusion of the left main coronary artery (black arrowhead). B: Supraaortic obstruction to outflow. C: Interference of mechanical valve leaflets.
Much of the approach to percutaneous PVL closure depends on the position of the valve involved, location of the leak, the presence of mechanical valves hindering entry, and the vascular access difficulties of the patient. In addition, the characteristics of the PVL can pose a challenge, such as calcification, a serpiginous tract, and orifice size differentials on each side of the prosthesis. The angles of approach can further add complexity. For mitral PVLs, the angulation of catheters and delivery sheaths to reach and cross the septal and posteriorly located leaks can be significant (Figure 3). Alternative approaches may be required for successful closure, including the use of combined approaches with the need for an arteriovenous rail. In the series by Cruz-González et al,4 a large percentage of patients (nearly 60%) had their mitral PVLs closed using a retrograde transaortic approach. In cases of mitral PVL with a 2-disc aortic prosthesis, it required crossing the central opening of the prosthesis with a hydrophilic glidewire and catheter and subsequent creation of an arteriovenous rail to provide the support necessary to advance the delivery sheath. This is a novel approach, previously reported by the same group, which has shown success.11 Nevertheless, this approach has inherent risks that include hemodynamic compromise during the procedure and the potential for damage to the mechanical discs or hinges.
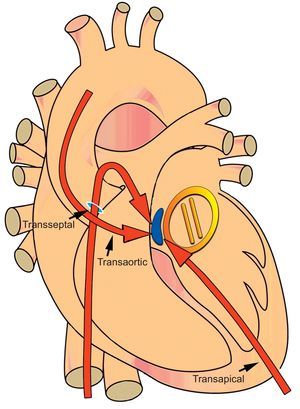
Diagrammatic representation of a paravalvular leak (blue) around a mechanical mitral valve (yellow) at the septal location (1-3 o’clock). Septal and posteriorly located leaks (1-6 o’clock) require step angulations of catheters and delivery sheaths from the antegrade transseptal and retrograde transaortic approaches. A percutaneous transapical approach provides direct access to both the aortic and mitral valves and reduces sharp catheter angulations and the need to transverse mechanical valves.
Alternative access methods such as transapical access provide an additional approach when traditional access methods (aortic: retrograde transaortic, antegrade transeptal; mitral: antegrade transseptal, retrograde transaortic) are unsuccessful or are a challenge due to technical issues. Based on our center's experience, we would argue that the primary approach to mitral PVLs, especially those located in the septal and posterior locations (1-6 o’clock position), is transapical. Percutaneous transapical (pTA) provides a direct approach to both the aortic valve and mitral valve apparatus and has been shown to significantly decrease procedural and fluoroscopy times.2 Percutaneous techniques for access and exit can obviate the need for direct surgical exposure while entry into the left ventricle can reduce sharp catheter angulations and the need to traverse mechanical valves (Figure 3).
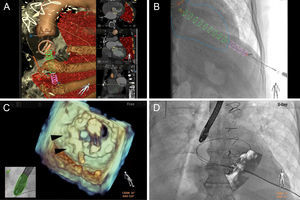
It is, however, multimodality imaging that aids in making successful and safe pTA PVL closure possible. The ability to integrate preprocedural computed tomographic angiography and intraprocedural transesophageal echocardiography, also known as fusion imaging, now offers an alternative to conventional, fluoroscopy-guided interventions. The use of computed tomographic angiography-fluoroscopy fusion imaging for pTA allows the route and site of entry to be determined such that it is aligned with the PVL and away from lung parenchyma, coronary arteries, and papillary muscles (Figure 4A).2 Despite cardiac motion, pTA can be less than 5mm away from the intended puncture site while maintaining a safe distance (1-2cm) away from the left anterior descending artery.12 On the other hand, echofluoroscopy fusion imaging relies on the use of transesophageal echocardiography data and merges it with fluoroscopy.13 Unlike computed tomographic angiography-fluoroscopy fusion, transesophageal echocardiography-fluoroscopy uses the real-time live data of both modalities, reducing many of the limitations of cardiac motion, patient positioning, and physiologic variations between timing of preprocedural studies. For PVL closure, superior visualization with 3D transesophageal echocardiography and confirmation with color Doppler assist in localizing the leak (Figure 4B). Landmarks can be placed at the center or margins or the shape of the entire leak can be drawn. Once registered and overlayed onto fluoroscopy, an appropriate c-arm view can be chosen to guide crossing the PVL. This technique can be particularly helpful with prosthetic valves that have minimal fluoroscopic markers.
Computed tomographic angiography-fluoroscopy (HeartNavigator, Philips HealthCare, Best, The Netherlands) and transesophageal echocardiography-fluoroscopy (EchoNavigator, Philips HealthCare, Andover MA) fusion imaging used for procedural guidance. A: Preprocedural planning with computed tomographic angiography-fluoroscopy fusion involves segmentation of the mechanical mitral and bioprosthetic aortic valves, aorta and ribs (all brown) and lungs (red). Landmarks are placed at the skin (red dot) and left ventricular apex (green dot) for transapical access, interatrial septum for transseptal access (blue dot), and the paravalvular leak (red dot/text). B: Registration and overlay of computed tomographic angiography-fluoroscopy onto fluoroscopy displays a cylinder (pink/green) that directs needle access into the left ventricle (outlined blue). C: Transesophageal echocardiography-fluoroscopy fusion shows a 3-dimensional en face view of a mechanical mitral valve in the open position with 2 Amplatzer vascular plug IIs (black arrowheads) located within the paravalvular leak and a safety wire still in place. D: Overlay of the transesophageal echocardiography onto live fluoroscopy reveals the transapical sheath across the paravalvular leak around the mitral valve, with a safety wire in place.
Overall, the treatment of PVLs is developing; endovascular approaches have grown substantially since the first closure by Hourihan et al in 1992.14 The choice of closure devices, although off-label, have shifted as our understanding of PVL characteristics and the potential for complications has increased. In addition, taking advantage of alternative access approaches, such as percutaneous transapical, and using multimodality imaging, specifically fusion imaging, now provides the potential to further advance this transcatheter procedure. As both patient and procedural complexity continue to escalate, rethinking transcatheter procedures, such as PVL closure, have increased the use of this once daunting procedure and made it more readily available for even the highest risk patients.
CONFLICTS OF INTERESTChad Kliger receives speaking honoraria from St Jude Medical. Carlos E. Ruiz is a consultant for St. Jude Medical, Valtech, Sorin, MediValve; advisor for St. Jude Medical; receives research grants from Philips Healthcare; has equity in Vascular Therapies, MitrAssist, Entourage, and BioInspire.