Patent foramen ovale (PFO) occurs when the septum primum and secundum fail to fuse after birth. A quarter of adults have this defect, which is usually an incidental finding with no clinical repercussions.1 However, the presence of PFO has been associated with a range of clinical conditions such as cryptogenic stroke, migraine, platypnea-orthodeoxia syndrome, and decompression illness.
Cryptogenic stroke accounts for up to 40% of all ischemic strokes.2 There has been heated debate about the role of percutaneous closure of PFO in patients with cryptogenic stroke. Recently, 3 randomized studies have been published (CLOSURE3, RESPECT4, and PC-trial5). None of these demonstrated that percutaneous closure was associated with a decreased incidence of stroke compared to medical treatment with antiplatelet agents or anticoagulants. Nevertheless, some subanalyses and metaanalyses of these studies have shown that percutaneous closure of PFO could be beneficial for certain patient groups.4,6–9
PATENT FORAMEN OVALE: DEFINITIONS AND DIAGNOSISThe PFO plays an important role in fetal circulation, whereby oxygenated blood from the umbilical veins and inferior vena cava flow through the Eustachian valve and the foramen to the left atrium and into systemic circulation.
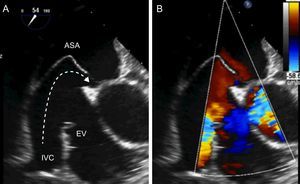
The morphology of PFO is variable and certain anatomic features such as large defects (> 5mm), persistent right-to-left shunt at rest, atrial septal aneurysm (ASA), and presence of a prominent Eustachian valve have been associated with a greater risk of paradoxical embolism (Figure 1).1,4 ASA is defined as the atrial septal membrane protruding > 10mm from the septal plane.1,4 ASA is found in approximately 35% of patients with PFO.4
Patent foramen ovale with high-risk anatomy for paradoxical embolism. A, patent foramen ovale with atrial septal aneurysm and a prominent Eustachian valve that guides flow from the inferior vena cava to the patent foramen ovale, leading to patency. B, right-to-left shunt through the patent foramen ovale. ASA, atrial septal aneurysm; EV, Eustachian valve; IVC, inferior vena cava.
Transthoracic echocardiography is the most commonly used type of diagnostic method for PFO. Given that color Doppler study only detects 5% to 10% of atrial shunts, intravenous injection of agitated saline solution increases diagnostic sensitivity. Microbubbles observed in the left atrium after 3 heart beats are indicative of extracardiac shunt. Injection is performed with the patient at rest and with a Valsalva maneuver. The main limitations of transthoracic echocardiography are its lower sensitivity compared to transesophageal echocardiography and its inability to provide detailed information on septal morphology.
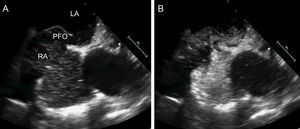
Transesophageal echocardiography is recommended if the findings of transthoracic echocardiography are negative or inconclusive but there is a high suspicion of PFO. In fact, most hospitals use transesophageal echocardiography to rule out a cardioembolic stroke, given that this technique can detect not only PFO but also presence of spontaneous contrast in the left atrium, left atrial appendage thrombus, left ventricular thrombus, and complex atheromatous plaques in the aorta. In addition, quantification of the shunt and assessment of the morphology of the PFO are more precise with transesophageal echocardiography (Figure 2).
CRYPTOGENIC STROKEDiagnosis of cryptogenic stroke can only be made by ruling out other sources of stroke such as carotid artery disease or cardioembolism. Several diagnostic studies are therefore required. In addition to echocardiography and carotid ultrasound, other studies should be performed to further support diagnosis of cryptogenic stroke. For example, the possibility of thromboembolic substrate of venous origin should be assessed in patients with PFO. Thus, deep vein thrombosis should be ruled out with venous Doppler studies or imaging of the venous system with magnetic resonance imaging or computed tomography. Likewise, it is also recommended to perform coagulation tests, such as prothrombin time, activated partial thromboplastin time, antiphospholipid antibodies, fibrinogen, protein C and S, resistance to activated protein C, and antithrombin.
Another substrate that could favor the onset of cryptogenic stroke is the presence of endovascular leads (from a pacemaker or defibrillator) in patients with PFO. In fact, recent studies point to increased incidence of stroke and transient ischemic attacks (TIAs) in patients carrying these devices.10 In 6075 patients in follow-up for a mean (standard devitation) of 4.7 (3.1) years, stroke or TIA was observed in 8.2% of patients with PFO compared to only 2% of those without PFO (hazard ratio [HR]=3.49; 95% confidence interval [95%CI], 2.33-5.25; P<.0001).
Medical TreatmentThere is no established consensus on optimal treatment given that the comparative data for anticoagulants and antiplatelet agents are limited. However, up to 5% of patients with cryptogenic stroke will experience another ischemic event within the year despite optimal medical treatment.3,5 Likewise, the most recent randomized studies of percutaneous closure of PFO showed that, among patients receiving treatment, stroke or TIA recurrence occurred in 6.8% in the CLOSURE I trial3 and in 5.2% in the PC-trial.5
In the Warfarin-Aspirin Recurrent Stroke Study (WARSS),11 2206 patients were randomized to acetylsalicylic acid or warfarin. After 2 years of follow-up, there were no significant differences in the recurrence of stroke, death, or major bleeding. Likewise, no significant differences were observed for patients with PFO. Although there is no clear consensus, some groups recommend antiplatelet therapy (acetylsalicylic acid, 325mg/day) as the first-choice treatment and oral anticoagulation with vitamin K antagonists for patients with deep vein thrombosis or those in states of hypercoagulability.
Percutaneous Closure of Patent Foramen OvalePercutaneous closure of PFO is a relatively simple procedure with a low rate of complications (< 1%). Approach is generally via the femoral vein under fluoroscopic guidance with transesophageal or intracardiac echocardiography.
Prior to the publication of the only 3 randomized studies to date, evidence of efficacy of percutaneous closure of PFO was based on a small number of nonrandomized comparative studies, case reports, and metaanalyses of these published data.
The CLOSURE I study was published in 2012 and was the first randomized study to compare medical treatment (acetylsalicylic acid, warfarin, or both) with percutaneous closure in 909 patients with PFO and cryptogenic stroke or TIA.3 No significant differences were observed for the composite of stroke/TIA at 2 years, all-cause mortality at 30 days, or neurologic death at 2 years (5.5% with the device compared to 6.8% with medical treatment; P=.37), or for the rate of stroke at 2 years (2.9% vs 3.1%, respectively; P=.79). However, this study was criticized because of the low rate of closure (87%) and the high rate of device thrombosis (1.1%) and atrial fibrillation (6%). These results might be due to the device used, the STARFLEX occluder (NMY Medical), have played a role in these observations as it has been associated with a greater incidence of thrombosis and atrial fibrillation.
The PC-trial5 and RESPECT trial4 were published simultaneously in 2013. The first of these had a similar design to the CLOSURE I study and included 414 patients with PFO and ischemic stroke, TIA, or peripheral embolism. Patients were randomized to closure with the AmplatzerTM PFO Occluder (APO) (St. Jude Medical) or medical treatment. At 4 years, there were no significant differences in the composite endpoint of death, nonfatal stroke, and peripheral embolism (3.4% with the device vs 5.3% with medical treatment; P=.63). In comparison with the CLOSURE I study, the use of APO was associated with a high rate of effective closure (95.9%) and a lower incidence of atrial fibrillation (2.9%) and device thrombosis (0%).
The RESPECT study had stricter inclusion criteria, as patients with TIA were excluded.4 Thus, only patients with established stroke (symptoms lasting more than 24h or with imaging evidence of cerebral infarction in magnetic resonance imaging or computed tomography) were randomized. In total, 980 patients were randomized to percutaneous closure with the APO device or medical treatment. The medical treatment group included 4 treatment regimens: acetylsalicylic acid 325mg/day (46.5%), warfarin (25.2%), clopidogrel 75mg/day (14%), acetylsalicylic acid with dipyridamole 200mg every 12h (8.1%), and acetylsalicylic acid with clopidogrel (6.2%). The low rate of events in this population is notable; it took 7 years to record the first 25 events (recurrent stroke or death after the procedure). In the intention-to-treat analysis, no significant differences were observed after 9 events in the closure group and 16 in the medical treatment group (HR=0.49; 95%CI, 0.22-1.11; P=.08). Nevertheless, this difference was significant in the per-protocol analysis (6 events in the closure group vs 14 events in the medical treatment group; HR=0.37; 95%CI, 0.14-0.96; P=.03) and according to treatment received (5 events vs 16 events; HR=0.27; 95%CI, 0.10-0.75; P=.007). In addition, in the subgroup analysis, major benefit was observed in association with percutaneous closure for patients with substantial right-to-left shunt (>20 bubbles) and for those with ASA. In agreement with the PC-trial, the effective closure rate was 93.5% with APO and 3% with atrial fibrillation.
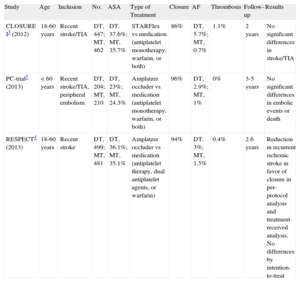
Four metaanalyses have been published since the results of these 3 studies have become available.9–12 Most of these metaanalyses show that, after grouping patients included in previous studies, percutaneous closure of PFO could be more effective than medical treatment for preventing recurrent thromboembolic events. One of the factors that could be important is the closure device used, as greater benefit was observed in a study that analyzed the results of APO separately (PC-trial and RESPECT).7 This finding could explain the higher rate of effective closure and the lower incidence of complications such as atrial fibrillation and thrombosis (Table).
Summary of Recent Randomized Studies of Percutaneous Closure of Patent Foramen Ovale
| Study | Age | Inclusion | No. | ASA | Type of Treatment | Closure | AF | Thrombosis | Follow-up | Results |
| CLOSURE I3 (2012) | 18-60 years | Recent stroke/TIA | DT, 447; MT, 462 | DT, 37.6%; MT, 35.7% | STARFlex vs medication (antiplatelet monotherapy, warfarin, or both) | 86% | DT, 5.7%; MT, 0.7% | 1.1% | 2 years | No significant differences in stroke/TIA |
| PC-trial5 (2013) | <60 years | Recent stroke/TIA, peripheral embolism | DT, 204; MT, 210 | DT, 23%; MT, 24.3% | Amplatzer occluder vs medication (antiplatelet monotherapy, warfarin, or both) | 96% | DT, 2.9%; MT, 1% | 0% | 3-5 years | No significant differences in embolic events or death |
| RESPECT4 (2013) | 18-60 years | Recent stroke | DT, 499; MT, 481 | DT, 36.1%; MT, 35.1% | Amplatzer occluder vs medication (antiplatelet therapy, dual antiplatelet agents, or warfarin) | 94% | DT, 3%; MT, 1.5% | 0.4% | 2.6 years | Reduction in recurrent ischemic stroke in favor of closure in per-protocol analysis and treatment received analysis. No differences by intention-to-treat |
AF, atrial fibrillation; ASA, atrial septal aneurysm; DT, device treatment; MT, medical treatment; TIA, transient ischemic attack;.
In 2015, recruitment to the REDUCE trial is expected to be complete. This fourth randomized study is comparing the Helex or GORE device (GORE Medical) with medical treatment. The inclusion criteria in the REDUCE trial are presence of PFO and stroke or cardioembolic TIA, assessed by a neurologist.
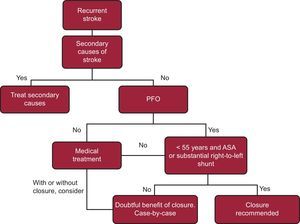
While waiting for the results of the REDUCE trial, management of patients with PFO and cryptogenic stroke remains the subject of debate, as there is no evidence to recommend systematic closure of PFO in all patients with cryptogenic stroke. Nevertheless, in some specific clinical situations, percutaneous closure of PFO could be justified. Examples include recurrent cryptogenic stroke in young patients (< 55 years) with evidence of venous thrombosis or high-risk anatomic features (severe right-to-left shunt, ASA, or Eustachian valve) (Figure 3).12
Algorithm for management of recurrent ictus with patent foramen ovale. ASA, atrial septal aneurysm; PFO, patent foramen ovale. According to Leong et al12.
Migraine is a chronic neurologic disease characterized by recurrent headache. The condition affects 8% to 13% of the adult population and is usually associated with autonomic symptoms or aura. Between 47% and 48% of patients with migraine have PFO, compared with between 17% and 20% of individuals in the general population.13
The MIST study was the first randomized study to assess closure of PFO with the STARFlex occluder to reduce recurrent migraine attacks.13 In total, 147 patients with PFO and migraine were randomized to closure of PFO or simulation of an intervention without closure. Follow-up of these patients lasted 3 to 6 months. Although a higher prevalence of right-to-left shunts was found in patients with migraine and aura, no significant differences were found between the 2 groups in terms of recurrent headache. The PREMIUM study is currently ongoing and will probably finish recruiting in 2014. The primary objective is to analyze the reduction in migraine attacks using the APO device. Until there is more evidence, at present there is insufficient support for systematic closure of PFO as beneficial for the treatment of migraine.
PLATYPNEA-ORTHODEOXIA SYNDROMEThis syndrome is a little-known condition that is hard to diagnose. The main clinical finding is dyspnea or hypoxemia when standing upright. These symptoms typically improve in decubitus. The syndrome usually occurs in elderly individuals and has been associated with aortic elongation and other anomalies such as pneumonectomy, pulmonary emphysema, and liver cirrhosis. These anatomic abnormalities can lead to vena cava displacement when standing. Thus, in patients with PFO the blood flow is directed towards the vena cava and right-to-left shunt occurs. Diagnosis is made by measuring arterial saturation at different points. A dynamic echocardiogram can also be recorded to demonstrate PFO. Definitive treatment of platypnea-orthodeoxia syndrome is percutaneous closure of the PFO. Success rates are close to 100% and the rate of complications is low.
DECOMPRESSION ILLNESSDecompression illness is uncommon. It is caused by the formation of air bubbles in circulation after substantial pressure changes. Divers, miners, noncommercial pilots, and even astronauts can be affected. The condition might not be as benign as was previously thought. A greater incidence of asymptomatic lesions has been observed in the brains of divers using computed tomography or magnetic resonance imaging. In patients with PFO, there is an increased risk of cerebral or spinal lesion. In divers with PFO, the risk of decompression symptoms and the risk of cerebral lesion in magnetic resonance imaging are higher. In addition, more pronounced defects are associated with increased risk of experiencing a more serious event, with symptoms lasting more than 24h, and with the need for treatment in a hyperbaric chamber.14,15
The only prospective controlled study in divers included 104 participants with a total of 18 394 dives during a period of 5.3 years (0.3) years. These participants were classified, without randomization, as those without PFO (39 divers), those with PFO who chose to undergo closure (26 divers), and those with PFO who chose not to undergo closure (39 divers). The divers with closed PFO had fewer episodes of symptomatic decompression illness and fewer cerebral ischemic lesions than those without closure.15
Given the current lack of evidence, we believe that systematic screening for PFO in all divers is not indicated. However, bearing in mind the consequences of an ischemic event, screening could be considered for professional divers, in view of their frequent dives, and for deep and technical divers, given the higher risk of decompression lesions. In any case, screening is always necessary after the appearance of decompression symptoms. Finally, percutaneous closure should be individualized according to presence of symptoms, presence of high-risk anatomic features together with PFO, type and frequency of dives, and desire of the patient to continue with the activity. The evidence supporting percutaneous closure of PFO in divers is poor, not because the studies were negative but because they were not randomized.
CONCLUSIONSPatent foramen ovale is a highly prevalent condition in adults (25%) and is associated with a greater incidence of cryptogenic stroke. Although statistical trends in favor of percutaneous closure have been reported in several studies, we currently do not have sufficient evidence to recommend systematic percutaneous closure of PFO in all patients with cryptogenic stroke. While we await the findings of the most recent randomized study (REDUCE), there are specific clinical situations, such as recurrent cryptogenic stroke in young patients (< 55 years) with evidence of venous thrombosis or high-risk anatomic features, in whom percutaneous closure could be justified.
CONFLICTS OF INTERESTDr. Freixa is a consultant for St. Jude Medical.