Obesity is an important cardiovascular risk factor and the location of fat deposits seems to be an important determinant of its metabolic impact. Visceral adipose tissue (VAT) exerts a harmful effect on metabolic homeostasis, but few longitudinal studies have evaluated the prognostic impact of the ratio of VAT to subcutaneous adipose tissue (SAT). This study aimed to evaluate whether the VAT/SAT ratio was associated with all-cause mortality and cardiac events.
MethodsRegistry-based retrospective cohort study. Eligible patients consisted of those without known heart disease referred to cardiac computed tomography (CT) angiography to evaluate suspected coronary artery disease (CAD). We included all patients with available information on VAT and SAT areas and coronary artery calcium (CAC) score. We assessed the combined endpoint of all-cause mortality, myocardial infarction or revascularization procedure at least 1 month after cardiac CT.
ResultsThe final population consisted of 713 participants (61% male; mean age, 57.7±10.2 years) followed up for a median of 1.3 years. The combined endpoint occurred in 66 patients; these patients showed a higher VAT/SAT ratio (1.06±0.74 vs 0.80±0.52, P=.0001). The VAT/SAT ratio was an independent predictor of death and cardiac events (HR = 1.43; 95%CI, 1.03-1.99), irrespective of cardiovascular risk factors, CAC, and the presence of CAD.
ConclusionsThe ratio between abdominal VAT/SAT was an independent predictor of death and coronary events, irrespective of cardiovascular risk factors, CAC, and the presence of CAD. This ratio is a CT-derived metric that may help to better identify patients with increased risk of death or cardiac events.
Keywords
Obesity is a challenging global epidemic with at least one third of the adult population being obese.1 It is associated with most cardiovascular diseases, including hypertension, coronary heart disease, and heart failure.2,3
The harmful impact of obesity is not only related to fat quantity but also to fat “quality” and distribution.4 Increased accumulation of fat in the abdomen, especially in the visceral compartment, is associated with metabolic risk factors and atherosclerosis.5,6 Visceral adipose tissue (VAT) is metabolically active by secreting adipokines, causing vascular inflammation and insulin resistance.7,8 VAT is associated with cardiovascular disease and represents a cardiometabolic risk marker.9 Prospective data from the Framingham Heart Study support the role of VAT as a predictor of mortality and cardiovascular disease,10,11 but longitudinal studies exploring this association are scarce and have limited external validity.12–15 In contrast, fat accumulation in the subcutaneous compartment is associated with a neutral or even beneficial metabolic impact.16 Therefore, the ratio of visceral to subcutaneous fat (VAT/subcutaneous adipose tissue [SAT] ratio) may provide a better assessment of the true cardiometabolic impact of body fat distribution, due to the differing systemic contributions of these anatomically close but functionally different fat depots.
This study aimed to evaluate whether the abdominal VAT/SAT ratio is associated with all-cause mortality and cardiovascular morbidity.
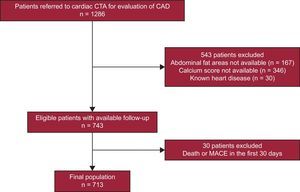
METHODSStudy ParticipantsThis was a registry-based retrospective cohort study using data from our Cardiovascular Diagnosis and Intervention Unit from a tertiary care hospital. The study sample was drawn from all patients who were referred for coronary computed tomography (CT) angiography for evaluation of coronary artery disease (CAD) from January 2008 to December 2013. Most patients (n=584) were referred without a previous ischemia test, and the remainder (n=129) had a previous inconclusive treadmill exercise test or single-photon emission CT scan. Only patients from our primary catchment area were considered for this study. We excluded patients with known cardiovascular disease (previous myocardial infarction, stroke or revascularization procedure, valvular heart disease, previous myocarditis or cardiomyopathy) or with serious life-threatening illness (life expectancy less than 1 year). The final population included patients with full available data on abdominal adipose tissue areas and coronary artery calcium (CAC) score who were followed up for a maximum of 3 years (Figure 1).
Patient selection flowchart. From the initial 1286 patients referred for cardiac CT due to suspected CAD, the final population included 713 patients. CAD, coronary artery disease; CT, computed tomography; CTA, computed tomography angiography; MACE, major acute cardiovascular events.
The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki and was approved by the institution's ethics committee. All participants gave their written informed consent to participate in the study.
Risk Factor AssessmentArterial hypertension was defined as history of hypertension (according to the European Society of Cardiology Guidelines17) or being on medical treatment with antihypertensive drugs. Smoking history was classified as never, former (participants who smoked for a minimum of 6 months during their lifetime but did not smoke at study entry) and current smoker. Type 2 diabetes mellitus was defined as a history of diabetes (according to current worldwide consensus criteria18) or being on medical treatment for diabetes. Family history of premature coronary heart disease was defined as having a first-degree relative who experienced a fatal or nonfatal myocardial infarction and/or coronary revascularization procedure before the age of 55 years in male relatives and 65 years in female relatives. Dyslipidemia was considered if the patient had a past history of dyslipidemia or was taking lipid-lowering drugs (statins, ezetimibe or fibrates).
Cardiac Computed Tomography Scan ProtocolAll patients underwent a cardiac multidetector CT scan in a 64-slice CT scanner (SOMATOM, Sensation 64, Siemens Medical Solutions, Forchheim, Germany) with 3 different acquisitions: the first for abdominal fat quantification, the second for CAC quantification and the third for coronary angiography. To assess abdominal fat, a single-slice abdominal CT scan was performed between L4 and L5, according to the method described by Borkan et al.19 The scan parameters were 120kV and 216mA with 5-mm thickness. This resulted in an estimated radiation exposure of 0.06 mSv. A blinded expert used the obtained slice to measure abdominal fat distribution: a cursor pointer was used to trace the VAT area by delineating the abdominal wall muscular layer20 and adipose tissue was identified in the areas with attenuation values ranging from−150 to−50 Hounsfield units (HU).21 Total abdominal fat area was measured, and the SAT area was obtained by subtracting VAT from the total abdominal fat area. As an estimate of the relative contribution of the VAT to the total abdominal fat, the VAT to SAT area was calculated (VAT/SAT ratio).
The following scan parameters were used to quantify the CAC: collimation, 24×1.2mm; gantry rotation time, 330ms; pitch, 0.2; tube voltage, 120kV; and tube current, 190 mAs. Image reconstruction of the calcium score acquisition was performed using an effective slice thickness of 3mm. CAC score was reported as the mean Agatston score and was calculated using a detection threshold of 130 HU using semiautomated software (Syngo Calcium Scoring, Siemens Medical Solutions) as described previously.22
Following CAC acquisition, CT angiography was performed (collimation, 64 x 0.6mm; tube current, 850 mAs; all other parameters similar to CAC acquisition scan). Tube current modulation with electrocardiographic pulsing for decreasing radiation dose was used, with full tube current applied at 60% to 65% of the RR interval. In patients with body weight lower than 70kg, tube voltage was reduced to 100kV. A bolus of 80 to 100mL of contrast (Ultravist, iopromide 370mg/mL, Bayer Schering Pharma AG, Berlin, Germany) was injected at 5mL/s via a power injector (Stellant D, Medrad Inc, Warrendale, Pennsylvania, United States) followed by a 40mL saline “chaser”, using a dedicated antecubital vein 18-gauge access catheter. A bolus-tracking technique was used, with a region of interest placed within the ascending aorta, set to detect a predefined threshold of 150 HU. For assessment of CAD, multiphasic sets of the CT reconstructed images were processed on a dedicated workstation (Aquarius Tera Recon Inc, San Mateo, California, United States) and analyzed for detection of at least 1 luminal diameter narrowing higher than 50% in any coronary artery segment by an experienced cardiologist. Severely calcified segments precluding lumen assessment were classified as positive for CAD.
Patient Follow-up and Combined EndpointAccording to our department's policy, only patients from our primary catchment area were followed up in order to collect information on major acute cardiovascular events (MACE). Patient follow-up data were collected by telephone interviews and electronic health record review at 12 and 36 months after cardiac CT. The combined endpoint included death from all causes, myocardial infarction or a revascularization procedure (percutaneous coronary intervention or coronary artery bypass graft surgery) at least 1 month after cardiac CT. Myocardial infarction was defined according to the most recent consensus definition.23 The decision to proceed to myocardial revascularization was made by the attending cardiologist/cardiac surgeon.
Statistical AnalysisContinuous variables are reported as mean±standard deviation or median (interquartile range). Discrete variables are expressed as frequencies and percentages.
An independent t test was used to assess differences in abdominal adipose tissue areas and the VAT/SAT ratio between participants according to the presence or absence of the combined endpoint.
Cox proportional hazards regression was used to perform the multivariate analysis using the following models: model 1 included age and sex; model 2 included age, sex, current smoking status, history of hypertension, type 2 diabetes mellitus, dyslipidemia, and familial history of premature CAD. Model 3 included all variables from model 2 plus coronary calcium score. A final model (model 4) was built including all variables from model 3 plus a dichotomic variable for presence/absence of CAD. The proportional hazards assumption of the Cox models was evaluated with Schoenfeld residuals.24 There was no evidence of departure from the assumption of proportionality. VAT, SAT, VAT/SAT ratio and CAC+1 were included in the models as a base-2 logarithm due to their skewed distribution (proportionality was assessed using the Kolmogorov-Smirnov test). One unit variation of the base-2 logarithmic transformation would be equivalent to a doubling of the variable of interest.
All statistical analyses were conducted using Stata 13.1 for Mac (StataCorp, College Station, Texas, United States).
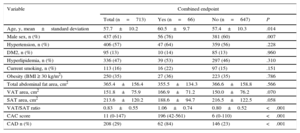
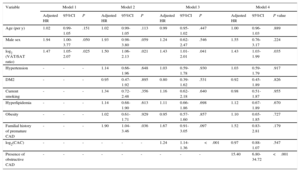
RESULTSParticipants’ Characteristics and Follow-up ResultsThe final population included 713 participants, 437 (61% men), with a mean age of 57.7±10.2 years. The participants’ characteristics according to the incidence of the combined endpoint during the follow-up period are depicted in Table 1.
Participants’ Characteristics
| Variable | Combined endpoint | |||
|---|---|---|---|---|
| Total (n=713) | Yes (n=66) | No (n=647) | P | |
| Age, y, mean±standard deviation | 57.7±10.2 | 60.5±9.7 | 57.4±10.3 | .014 |
| Male sex, n (%) | 437 (61) | 56 (76) | 381 (60) | .007 |
| Hypertension, n (%) | 406 (57) | 47 (64) | 359 (56) | .228 |
| DM2, n (%) | 95 (13) | 10 (14) | 85 (13) | .960 |
| Hyperlipidemia, n (%) | 336 (47) | 39 (53) | 297 (46) | .310 |
| Current smoking, n (%) | 113 (16) | 16 (22) | 97 (15) | .151 |
| Obesity (BMI ≥ 30 kg/m2) | 250 (35) | 27 (36) | 223 (35) | .786 |
| Total abdominal fat area, cm2 | 365.4±156.4 | 355.5±134.3 | 366.6±158.8 | .566 |
| VAT area, cm2 | 151.8±75.9 | 166.9±71.2 | 150.0±76.2 | .070 |
| SAT area, cm2 | 213.6±120.2 | 188.6±94.7 | 216.5±122.5 | .058 |
| VAT/SAT ratio | 0.83±0.55 | 1.06±0.74 | 0.80±0.52 | <.001 |
| CAC score | 11 (0-147) | 196 (42-561) | 6 (0-110) | <.001 |
| CAD n (%) | 208 (29) | 62 (84) | 146 (23) | <.001 |
BMI, body mass index; CAC, coronary artery calcium; CAD, coronary artery disease; DM2, type 2 diabetes mellitus; SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue.
Values are expressed as n (%), mean±standard deviation or median (interquartile range).
During a median follow-up of 1.3 years (interquartile range, 1.1-1.9 years) there were 18 deaths, 2 myocardial infarctions, and 54 revascularization procedures, for a total of 66 events of the combined endpoint. These participants showed a significantly increased VAT/SAT ratio (1.06±0.74 vs 0.80±0.52, P=.0001) and a trend to having a higher VAT area (166.9±71.2 vs 150.0±76.2 cm2) and lower SAT area (188.6±94.7 vs 216.5±122.5 cm2), which was statistically significant (Table 1).
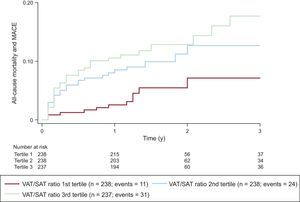
The total event rate was 6.0 (95% confidence interval [95%CI], 4.7-7.6) per 100 person-years of follow-up (1.2% in patients without obstructive CAD; 19.1% in patients with obstructive CAD in cardiac CT angiography). There was a significant increase in the event rate across the tertiles of the VAT/SAT ratio (3.0 events in the first tertile, 6.5 events in the second tertile and 8.5 events per 100 person-years for the last tertile, P=.0076). Figure 2 depicts the cumulative hazard curves of the combined endpoint over time according to the tertile of the VAT/SAT ratio.
Cumulative incidence of the combined endpoint over time according to the tertile of the VAT/SAT ratio. There was an increase in the event rate across the tertiles of the VAT/SAT ratio. MACE, major acute cardiovascular events; SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue.
In the age- and sex-adjusted analysis, doubling of the VAT/SAT ratio was associated with a 1.47 (95%CI, 1.05-2.07) increased hazard of the combined endpoint (Table 2). This association remained significant after adjustment for traditional cardiovascular risk factors (model 2, adjusted hazard ratio, 1.50; 95%CI, 1.06-2.13).
Multivariate Cox Regression Analyses for Evaluation of Log2 (VAT/SAT Ratio) as a Predictor of the Combined Endpoint (All-cause Mortality or Major Acute Cardiovascular Events)
| Variable | Model 1 | Model 2 | Model 3 | Model 4 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adjusted HR | 95%CI | P | Adjusted HR | 95%CI | P | Adjusted HR | 95%CI | P | Adjusted HR | 95%CI | P value | |
| Age (per y) | 1.02 | 0.99-1.05 | .151 | 1.02 | 0.99-1.05 | .113 | 0.99 | 0.95-1.02 | .447 | 1.00 | 0.96-1.03 | .889 |
| Male sex | 1.94 | 1.00-3.77 | .050 | 1.93 | 0.98-3.80 | .059 | 1.24 | 0.62-2.47 | .546 | 1.55 | 0.76-3.17 | .224 |
| log2 (VAT/SAT ratio) | 1.47 | 1.05-2.07 | .025 | 1.50 | 1.06-2.13 | .021 | 1.43 | 1.01-2.01 | .041 | 1.43 | 1.03-1.99 | .035 |
| Hypertension | - | - | - | 1.14 | 0.66-1.96 | .648 | 1.03 | 0.59-1.78 | .930 | 1.03 | 0.59-1.79 | .917 |
| DM2 | - | - | - | 0.95 | 0.47-1.92 | .895 | 0.80 | 0.39-1.62 | .531 | 0.92 | 0.45-1.89 | .826 |
| Current smoking | - | - | - | 1.34 | 0.72-2.48 | .356 | 1.16 | 0.62-2.18 | .640 | 0.98 | 0.51-1.87 | .955 |
| Hyperlipidemia | - | - | - | 1.14 | 0.68-1.90 | .613 | 1.11 | 0.66-1.86 | .698 | 1.12 | 0.67-1.89 | .670 |
| Obesity | - | - | - | 1.02 | 0.61-1.71 | .929 | 0.95 | 0.57-1.60 | .857 | 1.10 | 0.65-1.85 | .727 |
| Familial history of premature CAD | - | - | - | 1.90 | 1.04-3.46 | .036 | 1.67 | 0.91-3.05 | .097 | 1.52 | 0.83-2.81 | .179 |
| log2(CAC) | - | - | - | - | - | - | 1.24 | 1.14-1.36 | <.001 | 0.97 | 0.88-1.07 | .547 |
| Presence of obstructive CAD | - | - | - | - | - | - | - | - | - | 15.40 | 6.80-34.72 | <.001 |
95%CI, 95% confidence interval; CAC, coronary artery calcium; CAD, coronary artery disease; DM2, type 2 diabetes mellitus; HR, hazard ratio; SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue.
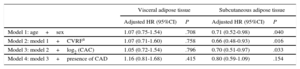
Visceral adipose tissue was not associated with the combined endpoint in the multivariate analyses (Table 3). However, higher SAT was independently associated with a lower risk of death or MACE irrespective of age, sex, cardiovascular risk factors, and CAC (HR, 0.70, 95%CI 0.51-.97, P=.033).
Multivariate Cox Regression Analyses for Evaluation of the Association Between Abdominal Visceral and Subcutaneous Adipose Tissues and the Combined Endpoint of All-cause Mortality or Major Acute Cardiovascular Events
| Visceral adipose tissue | Subcutaneous adipose tissue | |||
|---|---|---|---|---|
| Adjusted HR (95%CI) | P | Adjusted HR (95%CI) | P | |
| Model 1: age+sex | 1.07 (0.75-1.54) | .708 | 0.71 (0.52-0.98) | .040 |
| Model 2: model 1+CVRFa | 1.07 (0.71-1.60) | .758 | 0.66 (0.48-0.93) | .016 |
| Model 3: model 2+log2 (CAC) | 1.05 (0.72-1.54) | .796 | 0.70 (0.51-0.97) | .033 |
| Model 4: model 3+presence of CAD | 1.16 (0.81-1.68) | .415 | 0.80 (0.59-1.09) | .154 |
95%CI, 95% confidence interval; BMI, body mass index; CAC, coronary artery calcium; CAD, coronary artery disease; CVRF, cardiovascular risk factors; HR, hazard ratio.
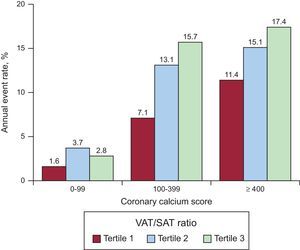
In the model including CAC score and traditional risk factors for CAD, a doubling of the VAT/SAT ratio was associated with a 1.43-fold increased hazard of the combined endpoint (95% CI, 1.01-2.01) (Table 2). The CAC and VAT/SAT ratio seemed to have an additive effect in the prediction of the combined endpoint of death or MACE (Figure 3).
Annual combined endpoint event rates stratified by coronary artery calcium score and tertiles of the VAT/SAT ratio. CAC and the VAT/SAT ratio seemed to have an additive effect in the prediction of the combined endpoint of death or MACE. CAC, coronary artery calcium; MACE, major acute cardiovascular events; SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue.
The prevalence of CAD was 29% (208 patients). The VAT/SAT ratio was a significant predictor of the combined endpoint (1.43-fold increased hazard of death or MACE per doubling of the VAT/SAT ratio) after adjustment for cardiovascular risk factors, CAC, and the presence of CAD (model 4, Table 2).
DISCUSSIONIn this study, including an adult population referred for cardiac CT angiography to evaluate CAD without known previous cardiovascular disease, we found that an increased abdominal VAT/SAT ratio was associated with a higher total mortality and incidence of MACE, independently of traditional vascular risk factors, CAC, and the presence of obstructive CAD. To our knowledge, this is the first longitudinal study to explore the influence of the relative distribution of abdominal fat between visceral and subcutaneous compartments on total mortality and incidence of cardiovascular events.
During the median follow-up of 1.3 years, there were 66 events of the combined endpoint (6.0 per 100 person-years of follow-up). Our results are in agreement with a meta-analysis on the association between CAD detected by CT angiography and cardiac events, including 32 studies and 41 960 patients over a mean follow-up of 1.96 years, reporting a composite MACE rate of 5.8% over the follow-up period.25 As expected, patients with coronary stenosis of at least 50% on cardiac CT angiography had a higher rate of MACE during the follow-up (19.1% vs 1.2% in patients without obstructive CAD).
Adipose tissue has been increasingly recognized as an important modulator of cardiovascular homeostasis. For example, during recent decades it was considered an abundant source of mesenchymal stem cells that produce several factors with angiogenic and immunomodulatory properties that might play a role in regenerative medicine targeting the heart.26 Furthermore, the effect of VAT and SAT upon cardiovascular health is still a matter of debate, with contradictory evidence, suggesting the need for a better biomarker for the impact of fat distribution on cardiovascular risk.27 According to the “portal vein hypothesis”,28 VAT is associated with increased delivery of free fatty acids to the liver and production of key inflammatory mediators, such as tumor necrosis factor-α and interleukin-6, leading to insulin resistance and systemic low-grade inflammation.29 On the other hand, the role of SAT in cardiometabolic risk is still controversial. Previous studies have shown that SAT may be associated with metabolic risk factors and increased insulin resistance, despite having a weaker association when compared with VAT.5,30 However, in overweight and obese patients, higher SAT is associated with insulin sensitivity,16 suggesting that in patients with increased body fat, the relative distribution in the abdominal compartment may be important to determine its global metabolic impact. In this study, VAT was not an independent predictor of the combined endpoint. However, our results showed that the SAT area was lower in participants who died or had a MACE during the follow-up, although this result was not statistically significant (P=.0584), and that higher SAT was associated with a lower risk of death or cardiac events after adjustment for age, sex, the presence of cardiovascular risk factors, and CAC. Our results support a protective role for SAT, which, according to previously published data, may be associated with improved insulin sensitivity and, therefore, a better metabolic profile.16
Visceral adipose tissue affects not only metabolic homeostasis but also cardiac function, especially diastole. Increased VAT is associated with impaired diastolic function both in asymptomatic persons31 and in those after a myocardial infarction.32 Its potential harmful impact upon cardiovascular health seems to be related to higher metabolic activity than SAT, with production of inflammatory mediators that generate a systemic proinflammatory state that represents a key mediator in the pathophysiology of heart failure with preserved ejection fraction.33 However, in our population, VAT was not a significant predictor of death or cardiac events. Nevertheless, even after adjustment for traditional risk factors, coronary calcium score and the presence of coronary stenosis of at least 50%, a higher VAT/SAT ratio predicted death and MACE during a median follow-up of 1.3 years. These findings support the recent “ectopic fat storage model” as the emerging paradigm of body fat contribution to metabolic risk.34 A dysfunctional SAT favors ectopic fat deposition in other compartments associated with insulin resistance and inflammation.35 In this way, the VAT/SAT ratio may provide a better index of the cardiometabolic impact of body fat composition than absolute quantification of each deposit independently.
The VAT/SAT ratio was a significant predictor of MACE and all-cause mortality irrespective of CAC score, a powerful and well-validated predictor of coronary events and mortality.36,37 Considering that CAC is an estimate of overall coronary plaque burden, we hypothesize that a higher VAT/SAT ratio may be associated with increased chronic low-grade systemic inflammation, predisposing patients to have vulnerable plaques, plaque rupture, and thrombosis,38 and therefore CAC and the VAT/SAT ratio seem to have an additive effect on the risk of death and cardiac events, as depicted in Figure 3.
LimitationsThe limitations of this study include the observational design and caution should be exercised when drawing conclusions about causality. All decisions to proceed to myocardial revascularization were made by the attending cardiologist and cardiac surgeon. We excluded revascularization procedures performed during the first month after coronary CT to avoid procedures that were prompted in the short-term by the result of the coronary CT angiography. Therefore, we can assume that most revascularization procedures were performed due to persistent angina despite anti-ischemic therapy or a positive ischemia test. Our cohort included a white population, so its external validity, especially to other ethnic groups, remains to be shown. Only patients from our primary catchment area were followed up to accurately collect information on MACE. Those patients would necessarily come to our center if they had a MACE, and therefore we tried to reduce to a minimum the potential loss of information on MACEs that would be managed in other centers. Only a small proportion of the initial sample underwent the 3-year follow-up (n=107). A sensitivity analysis (Table of the supplementary material) showed no significant differences in age, sex, cardiovascular risk factors, calcium score, areas of abdominal fat, and prevalence of CAD between groups according to the presence or absence of 3-year follow-up information. In addition, we did not systematically collect detailed information about the medical drugs patients were prescribed.
CONCLUSIONSThis study showed that the VAT/SAT ratio is an independent predictor of death and MACE. In addition to CAC quantification, this marker may become clinically relevant by providing a tool to better identify patients with an increased risk of death and cardiovascular disease.
- -
Obesity is a well-established cardiovascular risk factor and recent evidence has shown that its cardiovascular impact depends not only on its quantity but also on its location.
- -
In the abdominal compartment, VAT seems to have a deleterious impact on cardiovascular health, but the role of SAT is less well established. Furthermore, the association between the VAT/SAT ratio and cardiovascular disease has not been adequately explored.
- -
This retrospective study provides longitudinal data indicating that the ratio between VAT and SAT in the abdominal compartment is independently associated with all-cause death and MACE.
- -
This ratio may become a clinically relevant tool to better identify patients with an increased risk of death and cardiovascular disease.
None declared.