Significant tricuspid regurgitation (TR) is associated with increased morbidity and mortality. Clinical evaluation of TR patients is challenging. Our aim was to establish a new clinical classification specific for patients with TR, the 4A classification, and evaluate its prognostic performance.
MethodsWe included patients with isolated TR that was at least severe and without previous episodes of heart failure (HF) who were assessed in the heart valve clinic. We registered signs and symptoms of asthenia, ankle swelling, abdominal pain or distention and/or anorexia and followed up the patients every 6 months. The 4A classification ranged from A0 (no A) to A3 (3 or 4 As present). We defined a combined endpoint consisting of hospital admission due to right HF or cardiovascular mortality.
ResultsWe included 135 patients with significant TR between 2016 and 2021 (69% females, mean age 78±7 years). During a median follow-up of 26 [IQR, 10-41] months, 39% (n=53) patients had the combined endpoint: 34% (n=46) were admitted for HF and 5% (n=7) died. At baseline, 94% of the patients were in NYHA I or II, while 24% were in classes A2 or A3. The presence of A2 or A3 conferred a high incidence of events. The change in 4A class remained an independent marker of HF and cardiovascular mortality (adjusted HR per unit of change of 4A class, 1.95 [1.37-2.77]; P<.001).
ConclusionsThis study reports a novel clinical classification specifically for patients with TR that is based on signs and symptoms of right HF and has prognostic value for events.
Keywords
Tricuspid regurgitation (TR) is a prevalent valvular heart disease. Awareness of this traditionally forgotten valve has increased in the last few years, since the presence of significant TR has been associated with increased morbidity and mortality.1–4 Recent European Society of Cardiology guidelines recommend tricuspid valve (TV) surgery in symptomatic patients or in asymptomatic patients with progressive right ventricle (RV) dilatation or dysfunction.5 The New York Heart Association (NYHA) classification, which focuses mainly on symptoms of left heart failure (HF) such as the presence of dyspnea, is one of the main clinical tools for risk stratification in HF patients and has been used for almost a century. This classification is used for clinical assessment of patients included in clinical trials and observational studies in the field of TR but has not been specifically validated for this population. TR is associated with insidious symptoms due to the development of right-heart venous congestion and reduced forward stroke volume, in which dyspnea may not be the main symptom. A more specific clinical classification focused on the detection of systemic congestion may have prognostic implications and help identify patients who could benefit from an earlier intervention, since the optimal timing for intervention in isolated TR remains controversial and is often performed at a late stage of the disease.5
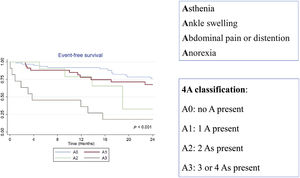
The aim of this study was to establish a new clinical classification, specific for patients with TR and based on signs and symptoms of right HF (4A: asthenia, ankle swelling, abdominal pain or distention, and anorexia) and to assess its prognostic value for events.
METHODSStudy design and patientsWe included consecutive patients with isolated TR that was at least severe and without previous episodes of HF who were assessed in our heart valve clinic. At our center, patients with significant TR assessed in the heart valve clinic undergo clinical evaluation, serum biomarker evaluation and a comprehensive echocardiogram on their first visit and every 6 months per clinical protocol while they remain clinically stable (follow-up may change if their clinical status deteriorates).
Exclusion criteria for all participants consisted of the need to start or increase diuretic treatment in the last 6 months, previous episodes of HF, the presence of significant (> mild) uncorrected left heart valve disease or alternative causes of right ventricular remodeling (previous RV infarction, arrhythmogenic RV cardiomyopathy, congenital heart disease or TR secondary to precapillary pulmonary hypertension in the context of lung disease and/or hypoxia).
In all patients, we recorded age, sex, and the presence of traditional cardiovascular risk factors. We also measured renal function, hemogram, B-type natriuretic peptide (BNP) levels and liver function enzymes.
Functional status was determined by the NYHA classification. We used a new clinical classification based on signs and symptoms of right HF.
Composition of the 4A classificationThis new clinical classification is based on signs and symptoms of right HF and considers the presence or absence of 4 different parameters:
- a)
Asthenia: presence of abnormal physical weakness or lack of energy reducing the ability to perform routine tasks.
- b)
Ankle swelling: presence of edema due to fluid retention in the ankles, feet or legs, not attributable to other causes of peripheral edema.
- c)
Abdominal pain or distention: subjective perception of abdominal pain, gastric fullness, or abdominal distention.
- d)
Anorexia: abnormal loss of appetite for food.
These parameters were established a priori. Despite their subjective component, they were chosen because they are simple and easy to assess during history-taking and physical examination in routine clinical practice. Before creating the model, we confirmed that each parameter was a marker of admission due to HF and mortality.
The 4A classification was built as follows:
- -
A0: no A present
- -
A1: 1 A present
- -
A2: 2 As present
- -
A3: 3 or 4 As present
A comprehensive transthoracic echocardiogram was performed in all patients (EPIQ system, Philips Medical Systems, United States). Parasternal, 3 apical (4-, 2- and 3-chamber views), and subcostal views were used to acquire 2-dimensional, color, pulsed, and continuous-wave Doppler data according to current recommendations.6–8 In our heart valve clinic, all studies are performed by the same operators and equipment to avoid variability.
Left ventricular (LV) ejection fraction was quantified using the Simpson biplane method and expressed as a percentage. Concomitant left-sided valvular heart disease and LV diastolic dysfunction were assessed according to current recommendations.6,9 RV assessment was performed on the RV focused 4-chamber view. In line with current recommendations, we measured RV fractional area change (FAC), tricuspid annular plane systolic excursion calculated using M mode, and RV Ś or systolic excursion velocity by Doppler tissue imaging. The severity of TR was evaluated according to current guidelines combining different semiquantitative and quantitative parameters.6
According to the new integrated classification of TR, etiology was divided into 3 main categories: primary TR, secondary TR, and TR related to a cardiac implantable electronic device. Primary TR was attributed to leaflet abnormalities. Secondary or functional TR was determined in the setting of nonleaflet abnormalities. These forms of TR were divided into atrial and ventricular: atrial TR was defined when right atrial enlargement and dysfunction leading to significant isolated annular dilation was the main cause of TR, usually in the context of older patients with permanent atrial fibrillation; ventricular TR was defined as the presence of RV enlargement and/or dysfunction leading to significant leaflet tethering and annular dilation associated with left heart disease (ventricular or valvular). Cardiac implantable electronic device-related TR was defined as that present mainly due to leaflet interaction with a cardiac implantable electronic device.10
The study protocol was reviewed and approved by the local institutional ethics committees. Informed consent was obtained. All procedures were carried out in accordance with the Declaration of Helsinki (2000).
Clinical outcomesFollow-up data included clinical status evaluated during clinical visits by NYHA and 4A classifications, all-cause mortality, cardiovascular mortality, hospital admission due to HF, TV surgery, and transcatheter valve intervention. Evaluation of RV size and function parameters was included, as were biomarker levels. We also considered a change in NYHA classification or a change in 4A classification, defined as a change in at least 1 step of the classification.
A combined endpoint of hospital admission due to right HF and cardiovascular mortality was defined.
Statistical analysisStatistical analysis was performed using SPSS software (version 21.0; SPSS, United States). The normality of distributions was tested with the Kolmogorov-Smirnov statistic. Categorical data are expressed as percentages, and continuous variables as mean±standard deviation or median [interquartile range], as appropriate. For comparisons of 2 normally distributed variables, the Student t test for continuous variables and the chi-square test for categorical variables were used, as appropriate. To create the model, we identified clinical variables that are used in every day clinical practice and the association between variables and events was confirmed. Multivariable Cox analysis was performed with a forward selection (likelihood ratio [LR]) modelling to determine independent associations with outcome (adjusted hazard ratio [HR] and 95% confidence interval [CI]), accounting for the rule of thumb for logistic and Cox models with a minimum of 10 outcome events per covariate. We limited our selection based on the biological plausibility of each variable, its relevance in current clinical decision-making, and the association with outcomes on univariate analyses. Event distribution according to the 4A classification and to each individual components of this classification were calculated according to the Kaplan-Meier method and compared by means of the log-rank test. Patients were censored at valve intervention.11 All tests were 2-tailed and P <.05 was considered significant.
RESULTSA total of 135 consecutive patients who met the inclusion criteria were assessed in the heart valve clinic for significant TR between 2016 and 2021. The patients’ demographic data and baseline characteristics are shown in table 1. Most of the patients were female and the mean age was 78±7 years. Atrial fibrillation was present in 87%. TR was primary in 9% (n=12), related to cardiac implantable electronic device in 4% (n=6), and secondary or functional in 87%: 44% secondary to previously corrected left-sided heart valve disease (these patients had developed TR after previous cardiac surgery and consequently, at the time of the surgery, concomitant TR surgery had not been indicated), and 39% had TR due to tricuspid annulus dilation in the context of atrial fibrillation (atrial TR). A total of 67% of the patients where under treatment with at least one type of diuretic, mainly loop diuretics (57%). Mean baseline values of laboratory data are also shown in table 1.
Demographic data and baseline characteristics
| Variable | All patientsN=135 |
|---|---|
| Age, y | 78±7 |
| Female sex | 93 (69) |
| Type 2 diabetes mellitus | 20 (15) |
| Hypertension | 79 (58) |
| Hypercholesterolemia | 60 (44) |
| Smoker | 15 (11) |
| Atrial fibrillation | 118 (87) |
| Coronary artery disease | 10 (7) |
| Previous cardiac surgery | 59 (44) |
| TR etiology | |
| Primary TR | 12 (9) |
| Functional TR | |
| Atrial TR | 53 (39) |
| Ventricular TR | |
| TR after previously corrected left valve disease | 60 (44) |
| TR in the context of nonvalvular left heart disease | 4 (3) |
| CIED-related TR | 6 (4) |
| Diuretic treatment | |
| At least 1 type | 90 (67) |
| Loop diuretics | 77 (57) |
| Thiazide | 3 (2) |
| Potassium-sparing diuretics | 42 (31) |
| Biochemistry | |
| Creatinine mg/dL, | 0.9±0.72 |
| Hemoglobin, g/dL | 12. 7±2 |
| BNP pg/mL | 141 [82-243] |
| Total bilirubin, mmol/L | 0.8 [0.6-1.2] |
| AST, U/L | 21 [18-25] |
| ALT, U/L | 16 [12-20] |
| GGT, U/L | 49 [27-93] |
| LDH, U/L | 229 [190-278] |
| ALP, U/L | 80 [67-98] |
| Echocardiography | |
| LV ejection fraction | 63±9 |
| LV end-diastolic diameter, mm | 43±7 |
| LV end-systolic diameter, mm | 26±6 |
| LV end-diastolic volume, mL | 62±23 |
| LV end-systolic volume, mL | 26±12 |
| RV basal diameter, mm | 51±5 |
| RV end-diastolic area, cm2 | 23±7 |
| RV end-systolic area, cm2 | 12±4 |
| RV FAC | 45±8 |
| TAPSE, mm | 21±4 |
| Śwave TDI, cm/sec | 10.2±2 |
| Pulmonary artery systolic pressure | 42±13 |
| Left atrium volume, mL/m2 | 66±54 |
| TR biplane vena contracta, mm | 0.9±0.4 |
| ERO, cm2 | [0.64±0.3] |
ALP, alkaline phosphatase; ALT, alanine transaminase; AST, aspartate transaminase; BNP, B-type natriuretic peptide; CIED, cardiovascular implantable electronic device; ERO, effective regurgitant orifice; FAC, factional area change; NYHA, New York Heart Association; LDH, lactate dehydrogenase LVEF, left ventricular ejection fraction; GGT, gamma-glutamyl transpeptidase; RV, right ventricle; TAPSE, tricuspid annular plane systolic excursion; TDI, tissue Doppler imaging; TR, tricuspid regurgitation; VC, vena contracta.
Values are expressed as No. (%), mean±standard deviation, or median [interquartile range].
According to echocardiographic parameters, most patients (83%) had severe TR, 13% had massive TR, and 4% had torrential TR. No significant differences were found in the severity of TR between patients in classes A1, A2 and A3 (Kruskal-Wallis P=.702). Mean values of the different parameters of RV and LV function are described in table 1. The baseline characteristics shown in table 1 did not differ between cohorts.
Follow-up and outcomesDuring a median follow-up of 26 [IQR, 10-41] months, 53 patients (39%) had the combined endpoint: 46 (34%) were admitted for HF and 7 (5%) died. During follow-up, 37 patients underwent tricuspid valve intervention (surgical correction in 23 and percutaneous correction in 14).
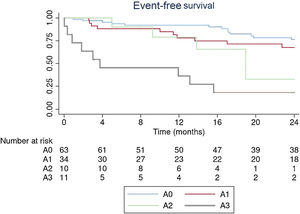
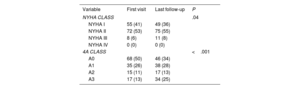
On baseline clinical evaluation, 94% of the patients were in NYHA I or II. In the 4A classification, 76% of patients were in A0 or A1. Differences in clinical status during follow-up are shown in table 2. In patients with events, the time between the change in 4A class and the event was >3 months.
Clinical status
| Variable | First visit | Last follow-up | P |
|---|---|---|---|
| NYHA CLASS | .04 | ||
| NYHA I | 55 (41) | 49 (36) | |
| NYHA II | 72 (53) | 75 (55) | |
| NYHA III | 8 (6) | 11 (8) | |
| NYHA IV | 0 (0) | 0 (0) | |
| 4A CLASS | <.001 | ||
| A0 | 68 (50) | 46 (34) | |
| A1 | 35 (26) | 38 (28) | |
| A2 | 15 (11) | 17 (13) | |
| A3 | 17 (13) | 34 (25) |
NYHA, New York Heart Association.
Values are expressed as No. (%).
Kaplan-Meier curves with event-free survival at 2 years according to the 4A classification are shown in figure 1. The presence of A2 or A3 showed a high incidence of events.
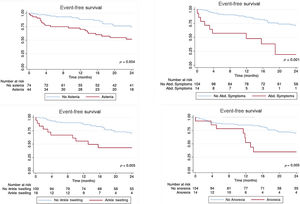
Event distribution according to each individual components of this classification, calculated according to the Kaplan-Meier method, is shown in figure 2. The global performance of the classification was evaluated using a c-statistic of 0.630.
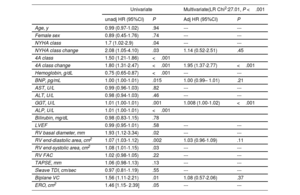
Univariate and multivariate Cox regression analysis of prediction of the outcome endpoint are shown in table 3. On univariate analysis, markers of the outcome endpoints were baseline 4A class and class change, NYHA class and class change, hemoglobin, BNP, gamma-glutamyl transpeptidase (GGT), alanine transaminase (ALP), RV diameter, and end-systolic and diastolic area, biplane vena contracta (VC), and effective regurgitant orifice. The change in 4A class and GGT remained independent markers of HF and CV mortality after adjustment for NYHA change, RV end-diastolic area, biplane VC, and BNP values (adjusted HR per unit of change of 4A class 1.95 [1.37-2.77], P <.001). The 4A classification for patients with TR is summarized in figure 3.
Results of univariate and multivariate analyses in prediction of the outcome endpoints
| Univariate | Multivariate(LR Chi2:27.01, P <.001 | |||
|---|---|---|---|---|
| unadj HR (95%CI) | P | Adj HR (95%CI) | P | |
| Age, y | 0.99 (0.97-1.02) | .94 | --- | --- |
| Female sex | 0.89 (0.45-1.76) | .74 | --- | --- |
| NYHA class | 1.7 (1.02-2.9) | .04 | --- | --- |
| NYHA class change | 2.08 (1.05-4.10) | .03 | 1.14 (0.52-2.51) | .45 |
| 4A class | 1.50 (1.21-1.86) | <.001 | ||
| 4A class change | 1.80 (1.31-2.47) | <.001 | 1.95 (1.37-2.77) | <.001 |
| Hemoglobin, g/dL | 0.75 (0.65-0.87) | <.001 | --- | --- |
| BNP, pg/mL | 1.00 (1.00-1.01) | .015 | 1.00 (0.99– 1.01) | .21 |
| AST, U/L | 0.99 (0.96-1.03) | .82 | --- | --- |
| ALT, U/L | 0.98 (0.94-1.03) | .46 | --- | --- |
| GGT, U/L | 1.01 (1.00-1.01) | .001 | 1.008 (1.00-1.02) | <.001 |
| ALP, U/L | 1.01 (1.00-1.01) | <.001 | ||
| Bilirubin, mg/dL | 0.98 (0.83-1.15) | .78 | ||
| LVEF | 0.99 (0.95-1.01) | .58 | --- | --- |
| RV basal diameter, mm | 1.93 (1.12-3.34) | .02 | --- | --- |
| RV end-diastolic area, cm2 | 1.07 (1.03-1.12) | .002 | 1.03 (0.96-1.09) | .11 |
| RV end-systolic area, cm2 | 1.08 (1.01-1.15) | .03 | --- | --- |
| RV FAC | 1.02 (0.98-1.05) | .22 | --- | --- |
| TAPSE, mm | 1.06 (0.98-1.13) | .13 | --- | --- |
| Śwave TDI, cm/sec | 0.97 (0.81-1.19) | .55 | --- | --- |
| Biplane VC | 1.56 (1.11-2.21) | .01 | 1.08 (0.57-2.06) | .37 |
| ERO, cm2 | 1.46 [1.15- 2.39] | .05 | --- | --- |
ALP, alkaline phosphatase; ALT, alanine transaminase; AST, aspartate transaminase; BNP, B-type natriuretic peptide; ERO, effective regurgitant orifice; FAC, factional area change; HR, hazard ratios; NYHA, New York Heart Association; LDH, lactate dehydrogenase LVEF, left ventricular ejection fraction; GGT, gamma-glutamyl transpeptidase; RV, right ventricle; TAPSE, tricuspid annular plane systolic excursion; TDI, tissue Doppler imaging; VC, vena contracta. Values are expressed as no. (%) or mean ± standard deviation.
For the first time, we show the use of a novel clinical classification, specific for patients with TR based on signs and symptoms of right HF and with prognostic value for events (hospital admission due to right HF and cardiovascular mortality).
The NYHA classification was first described in 1928 and has undergone several revisions since then.12 The classification is an easily applied first-line tool in everyday clinical practice that is widely used for the evaluation of symptoms of HF and clinical decision-making but is mainly based on symptoms of dyspnea on exertion.
The clinical status of patients with severe TR generally includes long asymptomatic periods because severe TR is relatively well tolerated. The result of low cardiac output may eventually lead to symptoms of asthenia, with abnormal physical weakness or lack of energy that may go unnoticed unless physicians specifically enquire about it, as well as symptoms related to the presence of elevated right atrial pressure such as peripheral edema, abdominal pain, distention, or early satiety.13,14 There is currently no clinical classification specific for patients who may develop symptoms related to right HF and that could be used easily during routine clinical practice, as patients may seem asymptomatic due to the absence of dyspnea but have insidious initial symptoms due to the presence of TR. The implementation of a new clinical classification specific for patients with TR, could help to identify patients at risk of events in an easy manner during routine clinical practice. As shown in our results, 94% of patients were in NYHA I or II at baseline, but 24% of the patients were already in A2 or A3. Using the 4A classification, we were able to identify patients in these classes and who were at higher risk of events. This could help identify those patients who could potentially benefit from an intervention, since the optimal timing for intervention in isolated TR remains controversial, and is often performed at a late stage of the disease.5 The use of the 4A classification during follow-up may alert us to the need for closer follow-up and additional testing or intervention, as a change in the 4A classification during follow-up showed prognostic impact.
Uncorrected severe TR is self-perpetuating and progressive and becomes highly disabling, with patients needing large amounts of diuretics. In the later stages, when systemic output is reduced, impaired organ function may be caused by elevated central venous filling pressure and reduced cardiac output, affecting mostly the kidney and liver. There are also implications for the gastrointestinal tract.15–17 The severity of TR has been associated with liver function abnormalities. Our results are in agreement with those other groups that have shown that the most prominent laboratory abnormalities are markers of cholestasis such as elevated bilirubin and GGT, rather than transaminase elevations and that these are independently associated with mortality.18–20
Biomarkers such as BNP levels are used in the identification and management of patients with left HF,21,22 but they are relatively nonspecific in patients with predominant right HF. In this study, multivariate analysis showed no prognostic impact of BNP levels in patients with severe TR. Specific gene expression patterns or microribonucleic acids (microRNAs) may help identify specific RV-biomarkers in future.23
Our findings show that the change in 4A class and GGT remained independent markers of HF and CV mortality after adjustment for NYHA change, RV end-diastolic area, biplane VC, and BNP values.
The large number of events during follow-up probably reflects the difficulties of managing this TR population, and the difficulties of understanding the natural history of TR. Patients at risk of HF or death could be identified earlier by systematic follow-up in a specific heart valve clinic applying an integrative approach that includes not only NYHA classification but also the 4A classification, liver and renal function, and novel RV parameters of dimension and function. This, in turn, could avoid taking actions at late stages of the disease.
The TRI-SCORE has recently been proposed; this model is a dedicated risk score model to predict in-hospital mortality in patients undergoing isolated tricuspid valve surgery. The model relies on 8 parameters, 4 clinical parameters (age ≥ 70 years, NYHA functional Class III-IV, right sided HF signs such as severe jugular venous distention, ascites, and/or marked peripheral edema, daily dose of furosemide ≥ 125 mg), 2 laboratory parameters (glomerular filtration rate <30mL/min, elevated total bilirubin), and 2 echocardiographic parameters (LV ejection fraction <60%, moderate/severe LV dysfunction).24 This score focuses on predicting the risk of mortality in patients undergoing surgery, whereas the 4A classification is a clinical classification that can be used during routine clinical practice for patient follow-up and decision-making.
LimitationsThis study has some limitations. First, this was an observational single-center study with a relatively small sample size that may limit its overall power. Larger prospective multicenter studies are needed for further validation of our findings. Our data were reported from clinical observations, and we did not systematically assess functional capacity or exercise tolerance with tests such as the 6-minute walk test or cardiopulmonary exercise test in all patients from baseline. However, unlike the prognostic role of these tests in patients with left HF, which has been thoroughly established, their role in patients with TR has been less well studied.
CONCLUSIONSThe use of the 4A classification, specific for patients with TR and based on signs and symptoms of right HF, has been shown to be prognostic of events (hospital admission due to right HF and CV mortality). Patients in NYHA I and II were already in class A2 or A3, and the presence of A2 or A3 showed a high incidence of events. The change in 4A class was an independent marker of HF and CV mortality. This new classification could therefore be of use during follow-up and decision-making in patients with significant TR, a scenario where tools for clinical management are scarce.
FUNDINGThere are no funding sources to disclose.
AUTHORS’ CONTRIBUTIONSThe authors confirm their contributions to the article as follows: study conception and design: A. González-Gómez, C. Fernández-Golfín, R. Hinojar, JM. Monteagudo, JL Zamorano; data collection: A. González, R. Hinojar, C. García-Sebastián; analysis and interpretation of results: A. González-Gómez, C. Fernández-Golfín, R. Hinojar, J. M. Monteagudo, A. García, J. L. Zamorano, Inés García-Lunar, Á. Sánchez-Recalde, L. Salido, A. Pardo; draft manuscript preparation: A. González-Gómez, C. Fernández-Golfín, R. Hinojar, J. M. Monteagudo, A. García, J.L. Zamorano. All authors have reviewed the results and approved the final version of the manuscript.
CONFLICTS OF INTERESTThere are no conflicts of interest to disclose.