Cardiac resynchronization therapy (CRT) is a well-established treatment for symptomatic heart failure patients with reduced left ventricular ejection fraction, prolonged QRS duration, and abnormal QRS morphology. The ultimate goals of modern CRT are to improve the proportion of patients responding to CRT and to maximize the response to CRT in patients who do respond. While the rate of CRT nonresponders has moderately but progressively decreased over the last 20 years, mostly in patients with left bundle branch block, in patients without left bundle branch block the response rate is almost unchanged. A number of technological advances have already contributed to achieve some of the objectives of modern CRT. They include novel lead design (the left ventricular quadripolar lead, and multipoint pacing), or the possibility to go beyond conventional delivery of CRT (left ventricular endocardial pacing, His bundle pacing). Furthermore, to improve CRT response, a triad of actions is paramount: reducing the burden of atrial fibrillation, reducing the number of appropriate and inappropriate interventions, and adequately predicting heart failure episodes. As in other fields of cardiology, technology and innovations for CRT delivery have been at the forefront in transforming–improving–patient care; therefore, these innovations are discussed in this review.
Keywords
Cardiac resynchronization therapy (CRT) is a well-established nonpharmacological treatment for symptomatic heart failure (HF) patients with reduced left ventricular ejection fraction (LVEF), prolonged QRS duration, and abnormal QRS morphology. As recently reported by the ALTITUDE registry,1 the improved outcome of CRT patients observed over the last decade is related to greater adherence to pharmacological therapy and device therapy as recommended by clinical practice guidelines.2,3 According to the most recent ESC guidelines,2 there is evidence (class I–level of recommendation A) for CRT implantation in HF patients (New York Heart Association [NYHA] functional class II-IV) with a LVEF < 35%, and with a QRS duration of more than 150ms and a left bundle branch (LBB) block morphology. For HF patients with a QRS duration less than 150ms and/or non-LBB block morphology, evidence is less weighty.3
The ultimate goals of modern CRT are to improve the proportion of patients responding to CRT and to maximize the response to CRT in those patients who do respond. While the rate of nonresponders has moderately but progressively decreased over the last 20 years of clinical use of CRT, mostly in patients with LBB block, in patients without LBB block the response rate is almost unchanged. This is well demonstrated by the most recent RESPOND-CRT trial,4 which showed a clinical composite response rate of 76.8% in CRT patients with LBB block and only 66% in CRT patients without LBB block. Furthermore, the proportion of patients known as “super-responders” to CRT (patients who show almost complete normalization of ventricular function and volumes) has remained constant over time, still representing only about 30% of all CRT patients.
A number of technological advances (Table) have already contributed to achieve some of the objectives of modern CRT, and some of them will be discussed in the present article. They include novel left ventricular (LV) lead design, prolongation of device longevity, improvement of automatic pacing chamber selection, of atrioventricular (AV) delay and of ventriculo-ventricular (VV) timing intervals, a reduction of the frequency and number of appropriate and inappropriate interventions, and finally the development of algorithms for reliable detection of impending HF decompensation episodes.
Bullet-point Overview of Topics Discussed in This Review
| Patient-based advances: |
| • Increased adherence to medical therapy |
| The CRT clinic: |
| • Multidisciplinary follow-up |
| • Correct implementation of CRT guidelines |
| • Increased knowledge on the technology behind CRT |
| • Device-based algorithms to predict HF episodes |
| Reducing the burden of atrial fibrillation: |
| • AV junction ablation |
| • Catheter-based arrhythmia ablation |
| • Automated algorithms to reduce AF episodes |
| Device- and lead-based advances: |
| • Quadripolar lead design |
| • Multipoint pacing |
| • Automated AV and VV timing (SonR, Adaptive CRT) |
| • Enhancing the amount of BiV pacing |
| • Prolonged battery longevity |
| Shock-reducing strategies: |
| • Prolonged detection periods |
| • Antitachycardia pacing |
| • Agorithms to reduce the number of ICD shocks |
| Ongoing investigations: |
| • Endocardial LV pacing |
| • His bundle pacing |
| • Computer models to visualize LV activation |
AF, atrial fibrillation; AV, atrioventricular; BiV, biventricular; CRT, cardiac resynchronization therapy; HF, heart failure; ICD, implantable cardioverter-defibrillator; LV, left ventricular; VV: ventriculo-ventricular.
Several studies have confirmed the importance of targeting a late activated electrical/mechanical area of the left ventricle for LV pacing.5,6 The design, shape and characteristics of LV pacing leads have considerably evolved during the past decades, driven by the clinical need to match a wide variety of cardiac vein anatomies while ensuring high mechanical stability and electrical performance. Left ventricular leads evolved from a single-electrode lead to the current quadripolar-electrode lead design. This important technological advances in LV lead design, coupled with major changes in device architecture output (independent programming of each LV pacing pole) and improvement in battery longevity, have made LV multipoint pacing (MPP) possible. The main advantage of MPP is that there is no need to implant multiple leads, avoiding a consequent increase in procedural complications (eg, lead dislodgment, wall perforation or lead fracture).
The modern quadripolar lead design enables “electronic repositioning”, ie, the possibility to select different pacing vectors and/or to adjust pacing output to ensure LV capture, while still avoiding phrenic nerve stimulation. Phrenic nerve stimulation and high LV capture threshold have been the most common reasons for repeated LV lead revisions with LV bipolar leads.7 Numerous reports on the safety and efficacy of LV quadripolar leads have indicated implant success rates as high as 98%, long-term data reporting stability performance in terms of pacing threshold and satisfactory dislodgement rates as low as 3%.8 Recently, Turakhia et al.,7 using a large USA retrospective cohort of patients with newly implanted CRT systems, compared patient survival, lead deactivation, and lead replacement with quadripolar vs bipolar leads. They showed that the use of the quadripolar LV lead, compared with a bipolar lead, was associated with a lower risk of death, LV lead replacement, and LV lead deactivation–even after adjustment for patient characteristics. These findings were consistent in patients with a high and low percentage of biventricular pacing. These results also indicate greater effectiveness of CRT associated with quadripolar leads, which is possibly due to the fact that the use of a LV quadripolar lead helps to avoid in-scar pacing. Indeed, it is well known that implantation of an LV lead in an area of myocardial scar may be associated with slow conduction–or even conduction block–resulting in less hemodynamic improvement and a poor clinical outcome.9
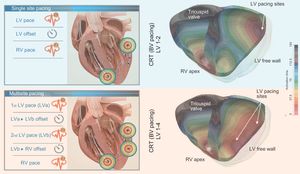
As indicated above, an additional advantage of the LV quadripolar lead is given by the potential delivery of stimulation from a single lead either simultaneously or sequentially through multiple electrodes (Figure). As the benefits of CRT are predominantly thought to result from improved LV electrical resynchronization, the concept of MPP has arisen as an alternative strategy for improving CRT success rates. Several clinical studies involving a limited number of patients have demonstrated an acute improvement in LV contractility and hemodynamic parameters including pressure-volume loops.10,11 Furthermore, MPP has been shown–in comparison with conventional biventricular pacing–to shorten QRS duration and LV activation time and to improve both mid- and long-term outcomes.12 However, these data conflict with the observations made in the most recent iSPOT (Left Ventricular Multispot Pacing for CRT) study. This trial showed that, in patients with LBB block, LV MPP has comparable improvement in contractility to the best conventional biventricular pacing.13 The chronic effect of MPP has been assessed mostly in small studies and only recently in a large prospective controlled trial (the MPP study).12 The primary efficacy endpoint of the MPP trial was met by demonstrating noninferiority of the response rate in the multipoint group compared with the conventional biventricular pacing group. Additional analyses demonstrated the ability of MPP technology to achieve an 87% response rate in patients with optimal program settings. More specifically, spatial separation between the different poles within the LV lead of more than 30mm and an activation delay between the different pacing site of 5ms conveyed the largest proportion of “super-responders” to CRT. Importantly, the MPP study preselected patients who were nonresponders to conventional CRT.14 At present, however, the clinical value of any multiple site pacing mode as default LV pacing mode is still not entirely clear.15,16 The fact that stimulating via additional pacing electrodes reduces device battery longevity should also be taken into consideration.
In silico representation of the effect of single-site pacing vs multipoint pacing. Schematic representation of left ventricular (LV) multisite pacing compared with single-site pacing (left panels). The right-sided panels represent an in silico patient-specific three-dimensional (3D) simulation of the activation pattern of the left and right ventricle. In the left upper panel, the conventional single-site LV pacing in combination with conventional right ventricular (RV) pacing is shown, while the right upper panel shows the resulting 3D BV activation map. In the left lower panel, a multipoint LV pacing setting is shown whereby the first LV pace (LVa) immediately precedes the second LV pace (LVb), creating a different activation pattern, depicted in the right lower panel. Multisite LV pacing allows for a better LV electrical synchronization as shown in the 3D simulation. BV, biventricular; CRT, cardiac resynchronization therapy.
In the near future, in silico patient-specific 3-dimensional simulations of the activation pattern of the left and right ventricle during different pacing modalities, and the use of multiple LV electrodes could be of help to fine tune ventricular activation (Figure) and, possibly, the resulting mechanical activation pattern.17
BEYOND CONVENTIONAL DELIVERY OF CRTIn the most recent years, a number of physiological considerations have challenged the concept of traditional delivery of CRT. The first of these concepts–LV endocardial pacing–is based on the observation that conventional CRT is delivered by placing a pacing lead at the endocardium of the right ventricle and one lead on the epicardial LV wall. This pacing configuration thus reverses the physiological LV activation (ie, from endocardium to epicardium).18,19 The second concept–His bundle pacing–is based on the observation that the block of the LBB is often very proximal in the AV conduction system, and that by pacing in the region of the His bundle with an appropriate lead and output, it is possible to restore normal conduction of the LBB.
LV Endocardial PacingOne of the available options to perform endocardial LV pacing is either with a conventional screw-in bipolar pacing lead implanted via a transseptal20,21 or transapical LV puncture. An alternative option to pacing from the endocardium is to place a wireless signal receiver in the LV cavity.22 Each of these approaches has some advantages and disadvantages represented by mitral valve insufficiency due to incomplete leaflet coaptation, increased risk of stroke due to a pacing lead permanently implanted in the LV, lead dislodgement, receiver detachment, and subsequent embolization.
The transapical puncture for LV pacing has only been anecdotally reported. In contrast, the feasibility, safety and clinical benefit of endocardial LV pacing via the transseptal approach have been investigated systematically. The largest experience with LV endocardial pacing is represented by the ALSYNC (ALternate Site Cardiac ResYNChronization) study.20 This study evaluated the feasibility and safety of LV endocardial pacing using a market-released pacing lead implanted via a single pectoral access by a novel atrial transseptal lead delivery system. ALSYNC was a prospective clinical investigational trial with a minimum 12-month follow-up in 18 centers where CRT-eligible patients, who had failed or were unsuitable for conventional CRT, were recruited. The LV endocardial lead was successfully implanted in 89.4% of the 138 enrolled patients. Freedom from complications meeting the definition of the primary endpoint was 82.2% at 6 months. In the study, 14 transient ischemic attacks (9 patients, 6.8%), 5 nondisabling strokes (5 patients, 3.8%), and 23 deaths (17.4%) were observed during the follow-up period. Not a single death resulted from a primary endpoint complication. At 6 months, the NYHA class improved in 59% of patients, and 55% of them had a reduction of the LV end-systolic volume of 15% or greater. The specific patient subgroup enrolled after a CRT nonresponse showed similar improvement with LV endocardial pacing.
An alternative option to perform endocardial LV pacing is leadless LV pacing. Two prospective studies–the Wireless Stimulation Endocardially for CRT Trial (WiSE-CRT)22 and the Safety and Performance of Electrodes Implanted in the Left Ventricle (SELECT-LV) study23–have investigated this novel and unique technology.22,23 Both studies included patients indicated for CRT who had “failed” conventional CRT or were nonresponders to conventional CRT. In the WiSE-CRT study, a successful system implantation was achieved in 13 patients (76.5%). Biventricular pacing was recorded in 83% and 92% of patients at 1 month and at 6 months, respectively. QRS duration was shorter during biventricular pacing compared with right ventricular pacing at 1 and 6 months. At the latter follow-up time point, two-thirds of patients had at least 1 functional NYHA class improvement. The LVEF significantly increased by 6 points at 6 months’ follow-up. The primary performance endpoint of the SELECT-LV study, biventricular pacing on the 12-lead electrocardiogram at 1 month, was achieved in 33 out of 34 patients. In that study, a total of 28 patients (84.8%) had improvement in the clinical composite score at 6 months, and 21 (66%) demonstrated a positive echocardiographic CRT response (> 5% absolute increase in LVEF). Serious procedure- or device-related events occurred in a limited number of patients (8.6%) within 24hours, and in 8 patients (22.9%) between 24hours and 1 month. Both the WiSE-CRT and the SELECT-LV study demonstrated clinical feasibility for the wireless LV lead system. This approach provided clinical benefits not only in patients with a standard indication for CRT, but in particular for those who did meet the criteria for a CRT upgrade, who were previously untreated, or who were considered to be a nonresponder—the “failed” CRT population. However, additional studies within postmarket surveillance registries or randomized controlled trials are needed to understand long-term outcomes, to compare additional outcomes, and to explore different techniques for selecting the optimal endocardial pacing site.
His Bundle PacingHis bundle pacing has been investigated in the past as a possible option to avoid the deleterious effects of long-term right ventricular pacing, but is now undergoing a revival in the context of CRT. In a subgroup of patients in whom the LBB block is located very proximally in the conductive system, His bundle pacing might be a favourable alternative option to restore the normal activation pattern.24 A distinction should be made between selective His bundle pacing vs nonselective His bundle pacing, ie, pacing in the proximity of the His bundle, presumably at high output. A recently published comparative study concluded that both selective His bundle pacing and high-output nonselective His-bundle pacing were able to restore normal electrical and LV mechanical synchrony. In 2015, a cross-over study in a limited patient population showed an equally beneficial response of His bundle pacing vs biventricular pacing.25 Furthermore, the technical possibility of placing a His bundle lead in lieu of the LV port of the CRT generator proved successful in terms of QRS narrowing and the achievement of resynchronization.26 Continued research is however needed to investigate the added value of this technique, especially to determine which patient subgroup would benefit the most from His-bundle pacing.
HOW CAN WE IMPROVE THE RESPONSE TO CRT?Among several factors that could adversely affect the response to CRT, suboptimal optimization of the AV delay and VV timing of the CRT device represents the most common–and supposedly the most readily correctable–variable.27 Several studies have demonstrated the acute hemodynamic benefits of optimization of AV and VV timings.28 Although echocardiography-guided optimization is an easily accessible method, it nevertheless remains a logistical challenging and resource-intensive process, with the programming parameters measured at rest and in the supine position. Therefore, device-based optimization is particularly appealing. However, studies using so-called “static” AV and VV-timing algorithms, ie, the selection of a given AV delay and/or VV timing programmed once at each follow-up visit, have reported neutral results compared with echocardiography-adjusted AV and VV timings. The failure of this “static” approach is most likely due to the inability to continuously adapt the AV delay and VV timing during physiological conditions such as exercise, but is also due to changes of the patient's underlying conduction properties.
Novel technology using the SonR contractility sensor or the Adaptive CRT algorithm could serve the prospect of an individualized, device-based strategy that can automatically optimize the AV and VV electrical timings, and the preferred paced chamber on a repetitive basis during rest and exercise. The clinical results achieved by using each of these 2 different “dynamic” algorithms point toward the superiority of this approach compared with echocardiographic optimization.
The SonR algorithm (LivaNova PLC, London, United Kingdom), consists of an accelerometer embedded in the tip of the right atrial electrode. The accelerometer records mechanical vibrations generated by the cardiac contraction which propagate through the entire heart. It provides a measure of cardiac contractility and serves as a basis for calculation of individual optimized AV- and VV-timing intervals. The sensor-based algorithm has been recently investigated in a large prospective randomized controlled trial4 and its safety and effectiveness have been compared with echocardiography-guided optimization of AV and VV timings. RESPOND-CRT4 was a prospective, randomized, double-blinded, multicenter, noninferiority trial. Patients were randomized (2:1, respectively) to receive weekly, automated CRT optimization with SonR vs an echocardiography-guided optimization of AV and VV timings. The primary efficacy endpoint was defined as the rate of clinical responders (patients alive, without adjudicated HF-related events, with improvement in NYHA class or quality of life) at 12 months. The study included 1039 patients. The rate of responders was 75.0% in the SonR group vs 70.4% within the control group (P < .001 for a noninferiority margin of 10.0%). At an overall mean follow-up of approximately 18 months, SonR was associated with a 35% relative risk reduction in HF hospitalizations (hazard ratio [HR], 0.65; 95% confidence interval [95%CI], 0.46–0.92; log rank test, P < .01). Patients with atrial fibrillation (AF) and patients with renal dysfunction allocated in the SonR arm showed a particularly large benefit compared with the control group.
The Adaptive CRT algorithm (Medtronic Inc, Minneapolis, Minnesota, United States) is a novel algorithm which, in the ambulatory setting, periodically measures intrinsic conduction and dynamically adjusts CRT pacing parameters. It minimizes the frequency of right ventricular pacing (hence improving the amount of LV or biventricular pacing). More specifically, if the conduction interval from the right atrium to the right ventricle is normal (AV delay ≤ 200ms during sinus rhythm), the algorithm provides LV-only pacing. The AV delay is then adjusted to produce an appropriate degree of fusion with the activation wave propagating through the still preserved portions of the His-Purkinje network.29 Indeed, early hemodynamic studies28,30,31 have demonstrated that in patients with normal AV conduction, LV pacing with fusion resynchronizes ventricular contraction better than simultaneous biventricular pacing. If the intrinsic AV conduction interval is, however, prolonged (AV > 200ms during sinus rhythm), the algorithm provides biventricular pacing. A more detailed discussion of the algorithm is provided elsewhere.29 This algorithm has been tested in a randomized controlled clinical trial with classic echocardiography-guided CRT optimization as a comparator. The percentage of synchronized LV pacing in the Adaptive CRT group was independently associated with a decreased risk of death or HF hospitalization, and with a significantly higher proportion of improved clinical outcomes at 6 and 12 months compared with the control group.32 Interestingly, however, a substudy by Yamasaki et al.33 found that the most considerable beneficial effects of the Adaptive CRT algorithm were present in a patient subgroup with LBB block and QRS morphology between 120 and 150ms, rather than in the patients with a wider QRS.
THE BURDEN OF ATRIAL FIBRILLATIONAlthough AF occurs in more than 25% of eligible CRT patients, the available evidence of benefit from CRT in patients with any type of AF is limited to observational trials or to registry data. The prognosis of HF patients with AF is generally worse than that of patients in sinus rhythm,34 and therapy with beta-blocking agents–although effectively reducing heart rate–does not impact on mortality.35
The use of CRT in HF patients who are either at risk of developing AF or already have a history of paroxysmal or permanent AF poses several questions and challenges. The influence of CRT on AF onset and burden has been considered rather modest so far. However, recent data from the previously described, randomized controlled trial on Adaptive CRT showed that patients receiving Adaptive CRT had a reduced risk of AF compared with those receiving conventional CRT.36 Over a mean follow-up period of 20.2 months, 8.7% of patients with Adaptive CRT and 16.2% with conventional CRT experienced the primary outcome (HR, 0.54; 95%CI, 0.31–0.93; P < .03). Most of the reduction in AF occurred in subgroups with prolonged AV conduction at baseline and with significant left atrial reverse remodelling. Moreover, AF burden and AF progression were significantly influenced by the Reactive antitachycardia pacing (ATP) algorithm.37,38 This novel ATP algorithm was successful in 44% of cases to terminate atrial tachycardial/AF episodes, especially in long atrial cycle lengths (> 210ms) and during atrial regular rhythms. Furthermore, the MINERVA (MINimizE Right Ventricular pacing to prevent Atrial fibrillation and heart failure) study showed a reduction in the progression of AF (48% reduced risk of persistent AF at 2 years vs standard pacing) and a reduced number of hospitalizations for AF. Taken together, the most recent results of the Adaptive CRT and MINERVA are extremely encouraging, since they indicate that the prognosis of CRT patients with paroxysmal or persistent AF can be further improved by proper and systematic adoption of device-based algorithms for AV-delay optimization or arrhythmia pacing termination. However, CRT patients with paroxysmal or persistent AF may also be offered the option of catheter-based ablation for AF. The CASTLE-AF (Catheter Ablation versus Standard conventional Treatment in patients with LEft ventricular dysfunction and Atrial Fibrillation) is a large prospective randomized controlled study including 397 patients randomized in a 1:1 fashion to conventional medical therapy or to pulmonary vein isolation (and eventually additional lines at the discretion of the operator).39 Cardiac resynchronization therapy patients undergoing AF ablation had a larger increment in LVEF, and a highly significant reduction in the composite endpoint of all-cause mortality and hospitalization for worsening of HF (HR, 0.62; 95%CI, 0.43-0.87; P = .007). Importantly, catheter ablation of AF in patients with “failed” CRT was also associated with improved cardiovascular mortality and hospitalization compared with conventional standard of care treatment.40
The effect of CRT is profoundly related to the continuous delivery of cardiac resynchronization, with a very strong “dose-effect” relationship. Even a few percentages of loss of cardiac resynchronization lead to a significant increase in mortality and risk of HF hospitalization. This was clearly demonstrated by Ousdigian et al.,41 who showed that a high percentage of biventricular pacing was not achieved in two-thirds of 8686 patients with persistent or permanent AF, and that these patients had an increased risk of death. However, one of the key issues is the documentation of effective delivery of CRT in patients with permanent AF. Kamath et al.42 showed that the percentage of biventricular pacing may be overestimated by CRT device-based counting algorithms. Therefore, a novel device-based algorithm for automatic detection of effective biventricular pacing has been developed, which uses the morphology of far-field intracardiac electrograms from the LV pacing cathode to distinguish between full capture and pseudofusion, and combined to another novel algorithm, which increases effective LV pacing during AF in patients undergoing CRT. This combined approach has been recently tested in the CRTee trial,43 which demonstrated that this novel device-based algorithm significantly increased the percentage of effective LV pacing during AF with only a minimal increase in heart rate. In some patients, the algorithm alone could increase the percentage of effective LV pacing to more than 95%. The simple noninvasive approach may prevent patients from undergoing further pharmacologic treatment and invasive ablation procedures. Despite all these algorithms, in some cases rate control is not sufficiently achieved. Although there are no randomized controlled trials evaluating the importance of performing AV junctional ablation in patients with AF and CRT–and the issue is still somewhat controversial–current guidelines strongly advocate ablation of the AV junction in CRT patients with permanent AF, a recommendation based upon evidence from large observational studies.44 However, the proper treatment strategy for CRT patients with different types of AF still remains to be unravelled in detail.
SHOCK-REDUCING STRATEGIES IN PATIENTS WITH CRT WITH DEFIBRILLATIONThe occurrence of both appropriate and inappropriate implantable cardioverter-defibrillator (ICD) shock is associated with a subsequent 3- to 5-fold increased risk of death among patients with primary prevention ICDs.45 Interventions aiming to reduce the number of shocks consist of the administration of antiarrhythmic drugs, device-programmed arrhythmia terminating algorithms, and ablation strategies. Data from the MADIT-CRT trial (Multicenter Automatic Defibrillator Implantation with CRT) showed that the administration of carvedilol was superior to metoprolol in terms of the prevention of inappropriate ATP and shock therapy (a 36% overall relative risk reduction, and even a 50% relative risk reduction if AF as a cause was considered separately).46 Moreover, superior outcomes with carvedilol vs metoprolol were observed for mortality, HF hospitalization, and ventricular tachycardia/ventricular fibrillation therapy overall.
Shock reduction strategies have included widespread use of empiric ATP for relatively rapid arrhythmias, strategic programming to delay ICD detection or treatment, and the development of improved arrhythmia discrimination algorithms. Two landmark trials in the prevention of shock therapy are the MADIT-RIT trial47 and the ADVANCE III study.48 A subsequent meta-analysis49 demonstrated that therapy reduction programming resulted in a 30% lower risk of death vs conventional programming. The reduction in mortality with therapy reduction programming was similar in all trials. Importantly, no significant difference in the risk of syncope or in the risk of appropriate shocks was observed with therapy reduction vs conventional programming. However, a 50% reduction in inappropriate shocks was found with therapy reduction vs conventional programming. These findings have been confirmed in a large “real-world” population of patients with CRT with defibrillation (CRT-D), the PainFree SST study,50 which included patients afflicted by a multitude of comorbidities including a significant proportion of patients with AF. Indeed, although 20% of CRT patients included in the Painfree SST study were receiving an ICD in secondary prevention and 37% of the CRT group had a history of atrial arrhythmias, the inappropriate shock incidence for CRT-D was one of the lowest reported in the literature so far: 1.5% at 1 year and 3.9% at 3 years of follow-up. Equally low was the incidence of any inappropriate therapy in CRT-D patients including shock and/or ATP therapy: 2.3% at 1 year, 3.7% at 2 years, and 5.7% at 3 years. All together, these data indicate that routine implementation of a predefined programming strategy in conjunction with the adoption of novel discrimination algorithms led to a very low rate of inappropriate shocks in CRT-D without increasing the risk of syncope.
THE PREDICTION OF EPISODES OF HEART FAILUREThe prediction of episodes of HF decompensation is an important goal from a patient's, physician's and health care economics perspective. Most of the risk scores used so far to identify patients at risk for the development of HF or mortality, assess a static risk at baseline or in an in-hospital setting. In contrast, implantable medical devices such as pacemakers, ICDs, and CRTs can provide daily measurements of multiple “diagnostic” parameters for possible evaluation of patients’ clinical status. Therefore, device-based prognosticators offer a great opportunity to develop a dynamic HF risk algorithm.
Earlier studies have shown that implantable device-based “diagnostics” such as intrathoracic impedance, AF burden and rate control information, nightly heart rate, heart rate variability, ventilation, and patient activity can identify when patients are at risk for HF events and could potentially be used unilaterally or in a combined manner for informed patient management. However, these studies have shown an overall low sensitivity and specificity to identify patients at risk of HF events and death.51 This inability to predict impending HF hospitalization is most likely due to the fact that a single (or a combination of few) sensor- or device-based diagnostics is unable to capture the multitude of pathophysiological processes that interact in a complex manner during HF and may culminate in a manifestation of acute decompensation.
The development and validation of a novel dynamic HF risk score derived from combining several diagnostic parameters monitored in implantable devices was first tested by Cowie et al.52 In a cohort of 2231 patients, these authors showed that the dynamic HF risk score developed in this study could identify when a high-risk patient is at a higher risk of impending HF hospitalization in an ambulatory setting, thus providing incremental information beyond what is provided by a static risk score. Unlike algorithms that conduct daily evaluation for the detection of worsening HF episodes, this analysis instead stratified patients into high-, medium-, and low-risk groups on the basis of monthly evaluations, demonstrating that the high-risk group had a > 5-fold risk of HF hospitalization compared with the low-risk group. A Bayesian model, again developed for monthly evaluation, identified patients with a 10-fold higher risk for hospitalization when comparing the high-risk group with the low-risk group, but it left nearly 40% of monthly evaluations in the middle group. A dynamic HF risk score is variable over time: the same patient may be at high and subsequently at low risk at different time periods depending on the status of continuously monitored device-based diagnostic parameters.
The most recent MultiSENSE53 confirmed the ability of a device-based algorithm to detect gradual worsening of HF over days or weeks. Overall, 900 patients (development cohort, n = 500; test cohort, n = 400) were followed up for up to 1 year. The HeartLogic index had a sensitivity of 70% with a median alert window of 34 days before most HF events occurred (defined as hospitalizations or outpatient visits with intravenous therapies as primary diagnosis), and an unexplained alert rate of only 1.47 per patient-year at the nominal threshold in the independent validation cohort. The HeartLogic multisensor index and alert algorithm provide a sensitive and timely predictor of impending HF decompensations. However, further studies are warranted to test the HeartLogic algorithm in larger HF populations.
CONCLUSIONS AND FUTURE PROSPECTSThe recognition by Carl Wiggers more than 90 years ago that conduction disturbances lead to LV dysfunction54 can be traced to experiments that provided the paradigm for CRT.55 Pacemaker technology, designed to correct ventricular conduction disturbances, was eventually tested in randomized, controlled CRT trials, driven by engineers, clinicians, and the industry. As probably in other fields of cardiology, technology and innovations for CRT delivery have been at the forefront in transforming–improving–patient care. Cardiac resynchronization therapy is a very unique example of a therapy that has united the fields of electrophysiology and HF, 2 distinct yet once very distant cardiology subspecialties. As knowledge and technology quickly evolve, novel ways to deliver CRT are already on the horizon and most likely will further reduce the rate of nonresponders.
CONFLICTS OF INTERESTA. Auricchio is a consultant to Medtronic, Boston Scientific, Biosense Webster, and LivaNova and has received speaker's fees from Medtronic, Boston Scientific, and LivaNova.
.