The management of elderly patients with chronic coronary syndrome (CCS) is challenging. We explored the prognostic value and usefulness for decision-making of ischemic burden determined by vasodilator stress cardiac magnetic resonance (CMR) imaging in elderly patients with known or suspected CCS.
MethodsThe study group comprised 2496 patients older than 70 years who underwent vasodilator stress CMR for known or suspected CCS. The ischemic burden (number of segments with stress-induced perfusion deficit) was calculated following the 17-segment model. Subsequently, we retrospectively analyzed its association with all-cause mortality and the effect of CMR-guided revascularization.
ResultsDuring a median follow-up of 4.58 years, there were 430 deaths (17.2%). A higher ischemic burden was an independent predictor of mortality (HR, 1.04; 95%CI, 1.01-1.07 for each additional ischemic segment; P=.006). This association was also found in patients older than 80 years and in women (P <.001). An interaction between revascularization and mortality was detected toward deleterious consequences at low ischemic burden and a protective effect in patients with extensive ischemia.
ConclusionsVasodilator stress CMR is a valuable tool to stratify risk in elderly patients with CCS and might be helpful to guide decision-making in this scenario.
Keywords
The prevalence, severity, and complexity of ischemic heart disease increase with age, largely due to aging-related factors such as comorbidities and other geriatric syndromes that affect the outcomes and clinical approach in these patients.1–3 These characteristics, intrinsic to the elderly population, often complicate diagnosis and can limit treatment options, negatively affecting the long-term prognosis.4,5
As a result of population aging, elderly patients with chronic coronary syndrome (CCS) represent a large proportion of the everyday clinical caseload.6,7 However, the evidence-based approach to diagnosis and treatment has been established from clinical trials, which underrepresent the older population.7
In the latest clinical guidelines, noninvasive imaging techniques play a key role in the treatment of CCS.8 Stress cardiac magnetic resonance (CMR) is an imaging technique with demonstrated clinical usefulness in this context, which provides a structural and functional assessment and allows detection and quantification of the extent of ischemia.9–11 However, despite the crucial importance of a correct understanding of ischemic heart disease in older patients, there is little evidence on the use of stress CMR in this context12 and its validity and applicability tend to be accepted as being similar to those in the general population.
We hypothesized that, in elderly patients with known or suspected CCS, more extensive ischemia, as determined by vasodilator stress CMR, would be predictive of all-cause mortality and help identify patients who would derive the greatest survival benefit from revascularization.
METHODSStudy populationOur study was based on a registry of consecutive patients referred for stress CMR study from the area served by our hospital between 2001 and 2016, with the indication of known or suspected CCS as per standard practice. It included 6700 patients of all ages, of which 311 were excluded. The reasons for exclusion and for performing stress CMR are detailed in figure 1 of the supplementary data. We recently used this registry to analyze the prognostic value and usefulness in revascularization decision-making of ischemic burden as identified on stress CMR in the whole registry group.13 From that cohort, we selected 2496 patients aged ≥ 70 years for the present study. The baseline patient characteristics and CMR details were collected prospectively in a predefined database. The CMR results were always available to the cardiologists managing the patients, and the treatment approach was selected according to the clinical judgement of the treating clinician.
Our registry was carried out in line with the principles of the Declaration of Helsinki and was approved by our institutional ethics committee, which in 2018 authorized a retrospective review of all-cause mortality for the patients included.
CMR protocolThe vasodilator stress CMR studies were carried out according to a previously-defined protocol9,11; the technical aspects are detailed in the supplementary data. All the studies were performed in a centralized manner and reported by 2 cardiologists accredited by the European Society of Cardiology and with more than 10 years of experience in the use and interpretation of stress CMR. If there were any uncertainties or difficulties in the interpretation, both operators reviewed the study, and the final results were decided by consensus.
Left ventricular ejection fraction (LVEF, %) and indexed left ventricular end diastolic volume (LVEDV) and end systolic volume (mL/m2) were quantified from cine images. The 17-segment model14 was used to define 2 postcontrast CMR indices. First, the ischemic load was defined visually as the number of segments showing a perfusion defect, which was established as a persistent delay in first-pass myocardial perfusion after vasodilator stress via the intravenous administration of dipyridamole (in at least 3 time-consecutive images compared with other segments in the same plane). Second, the extent of late gadolinium enhancement was defined as the number of segments that had an intensity signal on late gadolinium enhancement sequences> 2 standard deviations from a remote noninfarcted area and subsequently reviewed visually. The method and cut points used have been validated previously.9,11,13,15,16
Aims and follow-upThe primary aim was to assess the prognostic impact of ischemic burden on all-cause mortality over a long-term follow-up in a cohort of patients older than 70 years. The secondary aim was to explore the usefulness of ischemic burden in predicting the effect of stress CMR-guided revascularization on mortality.
CMR-guided revascularization was defined as any revascularization procedure, surgical or percutaneous, carried out within 3 months after stress CMR, provided that the patient had not been admitted to hospital for cardiovascular reasons during that time. This definition has previously been used by our group11,13 and other authors.17
Follow-up was carried out in a centralized manner using electronic clinical records, by 4 cardiologists authorized by the local ethics committee. The adjudication of events was by consensus, which aimed to confirm the event and its timing.
Statistical analysisStandard tests were used to assess the normality of the distribution of variables and compare normally-distributed data and nonparametric data. The association between ischemic burden and time to death was analyzed using Cox proportional hazards multivariate regression models. In each case, the risk ratio and corresponding 95% confidence interval (95%CI) were calculated.
To minimize potential selection bias, the effect of CMR-guided revascularization on all-cause mortality was analyzed for the whole study population and for a population that was 1:1 propensity-score matched, based on the individual probability of each patient being sent for revascularization (the model used can be seen in the table 1 of the supplementary data).
P values <.05 were considered statistically significant. Full information on the statistical analysis can be accessed in the supplementary data.
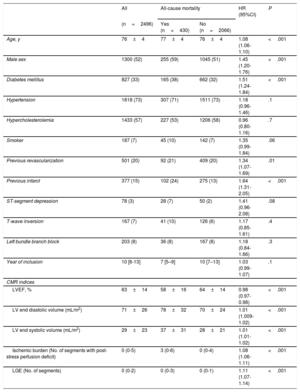
RESULTSIschemic burden and association with all-cause mortalityOver a median follow-up of 4.58 years (238 [range, 99-414] weeks), 430 deaths from all causes were registered (17.2%). The annual mortality, calculated as the number of deaths per 100 people over 1 year, was 3.3. The clinical and CMR characteristics are shown in table 1. All-cause mortality was associated with older age, male sex, and history of diabetes, acute myocardial infarction, and revascularization. Regarding the CMR indices, patients who reached the primary outcome had larger indexed left ventricular end systolic volume and LVEDV, lower LVEF, and more extensive ischemic burden and late gadolinium enhancement.
Clinical and CMR characteristics
| All | All-cause mortality | HR (95%CI) | P | ||
|---|---|---|---|---|---|
| (n=2496) | Yes (n=430) | No (n=2066) | |||
| Age, y | 76±4 | 77±4 | 76±4 | 1.08 (1.06-1.10) | <.001 |
| Male sex | 1300 (52) | 255 (59) | 1045 (51) | 1.45 (1.20-1.76) | <.001 |
| Diabetes mellitus | 827 (33) | 165 (38) | 662 (32) | 1.51 (1.24-1.84) | <.001 |
| Hypertension | 1818 (73) | 307 (71) | 1511 (73) | 1.18 (0.96-1.46) | .1 |
| Hypercholesterolemia | 1433 (57) | 227 (53) | 1206 (58) | 0.96 (0.80-1.16) | .7 |
| Smoker | 187 (7) | 45 (10) | 142 (7) | 1.35 (0.99-1.84) | .06 |
| Previous revascularization | 501 (20) | 92 (21) | 409 (20) | 1.34 (1.07-1.69) | .01 |
| Previous infarct | 377 (15) | 102 (24) | 275 (13) | 1.64 (1.31-2.05) | <.001 |
| ST-segment depression | 78 (3) | 28 (7) | 50 (2) | 1.41 (0.96-2.08) | .08 |
| T-wave inversion | 167 (7) | 41 (10) | 126 (6) | 1.17 (0.85-1.61) | .4 |
| Left bundle branch block | 203 (8) | 36 (8) | 167 (8) | 1.18 (0.84-1.66) | .3 |
| Year of inclusion | 10 [6-13] | 7 [5–9] | 10 [7–13] | 1.03 (0.99-1.07) | .1 |
| CMR indices | |||||
| LVEF, % | 63±14 | 58±16 | 64±14 | 0.98 (0.97-0.98) | <.001 |
| LV end diastolic volume (mL/m2) | 71±26 | 78±32 | 70±24 | 1.01 (1.009-1.02) | <.001 |
| LV end systolic volume (mL/m2) | 29±23 | 37±31 | 28±21 | 1.01 (1.01-1.02) | <.001 |
| Ischemic burden (No. of segments with post-stress perfusion deficit) | 0 (0-5) | 3 (0-6) | 0 (0-4) | 1.08 (1.06-1.11) | <.001 |
| LGE (No. of segments) | 0 (0-2) | 0 (0-3) | 0 (0-1) | 1.11 (1.07-1.14) | <.001 |
95%CI, 95% confidence interval; CMR, cardiac magnetic resonance; HR, hazard ratio; LGE, late gadolinium enhancement; LV, left ventricle; LVEF, left ventricular ejection fraction.
Values are expressed as mean±standard deviation, No. (%) or mean [range]. Year of inclusion: from 1 to 16, corresponding to 2001 to 2016.
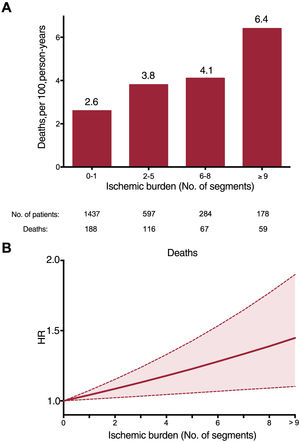
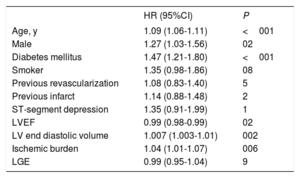
The annual all-cause mortality rate increased in parallel with increasing ischemic burden (figure 1A). After adjustment for the variables that were independently associated with death (age, male, diabetes, LFEDV and LVEF), a significant positive association was observed between the number of ischemic segments and the risk of death (hazard ratio [HR]=1.04; 95%CI, 1.01-1.07 for each additional segment with a post-stress perfusion defect; P=.006) (table 2 and figure 1B). The c-statistic was 0.60 [0.57-0.63] for ischemic burden as a predictor of mortality and was 0.66 [0.63-0.69] for the multivariate analysis model.
All-cause mortality. Multivariate analysis
| HR (95%CI) | P | |
|---|---|---|
| Age, y | 1.09 (1.06-1.11) | <001 |
| Male | 1.27 (1.03-1.56) | 02 |
| Diabetes mellitus | 1.47 (1.21-1.80) | <001 |
| Smoker | 1.35 (0.98-1.86) | 08 |
| Previous revascularization | 1.08 (0.83-1.40) | 5 |
| Previous infarct | 1.14 (0.88-1.48) | 2 |
| ST-segment depression | 1.35 (0.91-1.99) | 1 |
| LVEF | 0.99 (0.98-0.99) | 02 |
| LV end diastolic volume | 1.007 (1.003-1.01) | 002 |
| Ischemic burden | 1.04 (1.01-1.07) | 006 |
| LGE | 0.99 (0.95-1.04) | 9 |
95%CI, 95% confidence interval; HR, hazard ratio; LGE, late gadolinium enhancement; LV, left ventricle; LVEF, left ventricular ejection fraction.
The collinearity of the variables included in the multivariate model was assessed using the variance inflation factor: age, 1.02; male sex, 1.15; diabetes, 1.02; smoker, 1.03; previous revascularization, 1.27; previous infarct, 1.29; ST-segment depression; 1.02; LVEF, 2.25; LV end diastolic volume, 1.85; ischemic burden, 1.47; LGE, 2.09. LV end systolic volume was removed from the multivariate analysis due to excessive collinearity: tolerance <0.20; variance inflation factor> 5; correlation> 0.8 with LVEF.
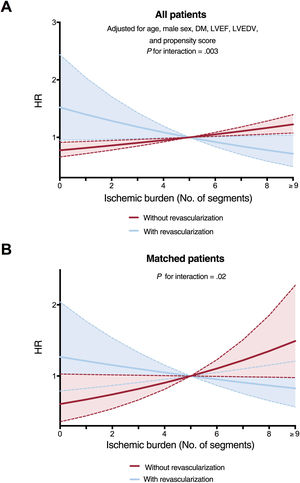
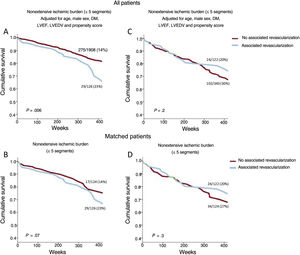
A cut point of 5 ischemic segments was used to stratify patients into categories of extensive and nonextensive ischemic burden. This was the best cut point, derived from the Youden index applied to the analysis of the receiver operating characteristic curve, which explores the association between extent of ischemia and total mortality. This cut point also coincided with the point where the effects of ischemic burden on all-cause mortality in patients who had undergone revascularization and those who had not were inverted, in both the analysis of all patients (figure 2A) and the propensity-score matched population (figure 2B).
Effect of extent of ischemic burden on all-cause mortality according to the use of stress CMR-guided revascularization. A: for the whole registry. B: in the matched population. CMR, cardiac magnetic resonance; DM, diabetes mellitus; HR, hazard ratio; LVEDV, left ventricular end diastolic volume; LVEF, left ventricular ejection fraction.
Patients with extensive ischemia (> 5 segments; n=462, 19%) had a higher annual mortality rate than those with nonextensive ischemia (≤ 5 segments; n=2034, 81%): 4.9 vs 2.9 deaths/100 person-years; P <.001). When this same analysis was performed for age subgroups (figure 2 of the supplementary data), the annual mortality rate was significantly higher in those with extensive ischemia, both for the group aged 70 to 80 years (n=1988; 4.5 vs 2.6 deaths/100 person-years; P <.001) and for the over-80 group (n=508; 7.4 vs 4.8 deaths/100 person-years; P <.001). Lastly, when we analyzed the association between the severity of ischemic burden and the variable of sex (figure 2B of the supplementary data), there was also a higher annual mortality rate in the group of patients with extensive ischemia, both in the male subgroup (n=1300; 5.4 vs 35 deaths/100 person-years; P <.001) and in the female subgroup (n=1196; 4.2 vs 2.5 deaths/100 person-years; P <.001).
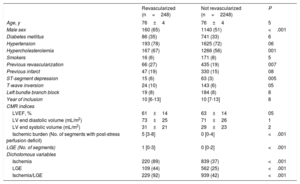
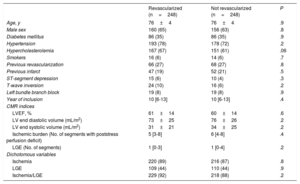
Association between ischemic burden and effect of CMR-guided revascularization on all-cause mortalityDuring follow-up, 248 patients were registered as having undergone CMR-guided revascularization (9.9%; 178 [72%] were percutaneous and 70 [28%] were surgical). The clinical and CMR characteristics of the patients who did (n=248) and did not (n=2248) undergo revascularization are shown in table 3. A propensity score for predicted use of CMR-guided revascularization was obtained; this model was used to select a 1:1 sample of patients with comparable characteristics who had and had not undergone revascularization (table 4). Revascularized patients with nonextensive ischemia (≤ 5 segments) comprised 126 of the 248 patients (99 percutaneous and 27 surgical), while the other 122 revascularized patients (79 percutaneous and 43 surgical) had extensive ischemia (> 5 segments). Ventricular function as determined by LVEF was not significantly different between the matched groups, either for patients without extensive ischemia (64±12 vs 65±13; P=.6) or those with extensive ischemia (57±15 vs 57±14; P=.9).
Clinical and CMR characteristics of patients who received and did not receive CMR-guided revascularization in the whole registry
| Revascularized (n=248) | Not revascularized (n=2248) | P | |
|---|---|---|---|
| Age, y | 76±4 | 76±4 | 5 |
| Male sex | 160 (65) | 1140 (51) | <.001 |
| Diabetes mellitus | 86 (35) | 741 (33) | 6 |
| Hypertension | 193 (78) | 1625 (72) | 06 |
| Hypercholesterolemia | 167 (67) | 1266 (56) | 001 |
| Smokers | 16 (6) | 171 (8) | 5 |
| Previous revascularization | 66 (27) | 435 (19) | 007 |
| Previous infarct | 47 (19) | 330 (15) | 08 |
| ST-segment depression | 15 (6) | 63 (3) | 005 |
| T-wave inversion | 24 (10) | 143 (6) | 05 |
| Left bundle branch block | 19 (8) | 184 (8) | 8 |
| Year of inclusion | 10 [6-13] | 10 [7-13] | 8 |
| CMR indices | |||
| LVEF, % | 61±14 | 63±14 | 05 |
| LV end diastolic volume (mL/m2) | 73±25 | 71±26 | 1 |
| LV end systolic volume (mL/m2) | 31±21 | 29±23 | 2 |
| Ischemic burden (No. of segments with post-stress perfusion deficit) | 5 [3-8] | 0 [0-4] | <.001 |
| LGE (No. of segments) | 1 [0-3] | 0 [0-2] | <.001 |
| Dichotomous variables | |||
| Ischemia | 220 (89) | 839 (37) | <.001 |
| LGE | 109 (44) | 562 (25) | <.001 |
| Ischemia/LGE | 229 (92) | 939 (42) | <.001 |
CMR, cardiac magnetic resonance; LGE, late gadolinium enhancement; LV, left ventricle; LVEF, left ventricular ejection fraction.
Values are expressed as mean±standard deviation, No. (%) or mean [range]. Year of inclusion: from 1 to 16 corresponding to the years 2001 to 2016. For dichotomous variables, ischemia or LGE were judged to exist when more than 1 segment had a perfusion deficit or LGE, respectively, according to the technical specifications detailed in the CMR study protocol.
Patient and CMR characteristics for those who received and did not receive CMR-guided revascularization in the matched population
| Revascularized (n=248) | Not revascularized (n=248) | P | |
|---|---|---|---|
| Age, y | 76±4 | 76±4 | .9 |
| Male sex | 160 (65) | 156 (63) | .8 |
| Diabetes mellitus | 86 (35) | 86 (35) | .9 |
| Hypertension | 193 (78) | 178 (72) | .2 |
| Hypercholesterolemia | 167 (67) | 151 (61) | .06 |
| Smokers | 16 (6) | 14 (6) | .7 |
| Previous revascularization | 66 (27) | 68 (27) | .8 |
| Previous infarct | 47 (19) | 52 (21) | .5 |
| ST-segment depression | 15 (6) | 10 (4) | .3 |
| T-wave inversion | 24 (10) | 16 (6) | .2 |
| Left bundle branch block | 19 (8) | 19 (8) | .9 |
| Year of inclusion | 10 [6-13] | 10 [6-13] | .4 |
| CMR indices | |||
| LVEF, % | 61±14 | 60±14 | .6 |
| LV end diastolic volume (mL/m2) | 73±25 | 76±26 | .2 |
| LV end systolic volume (mL/m2) | 31±21 | 34±25 | .2 |
| Ischemic burden (No. of segments with poststress perfusion deficit) | 5 [3-8] | 6 [4-8] | .4 |
| LGE (No. of segments) | 1 [0-3] | 1 [0-4] | .2 |
| Dichotomous variables | |||
| Ischemia | 220 (89) | 216 (87) | .8 |
| LGE | 109 (44) | 110 (44) | .9 |
| Ischemia/LGE | 229 (92) | 218 (88) | .2 |
CMR, cardiac magnetic resonance; LGE, late gadolinium enhancement; LV, left ventricle; LVEF, left ventricular ejection fraction.
Values are expressed as mean±standard deviation, No. (%) or mean [range]. Year of inclusion: from 1 to 16, corresponding to the years 2001 to 2016. For dichotomous variables, ischemia or LGE were judged to exist when more than 1 segment had a perfusion deficit or LGE, respectively, according to the technical specifications detailed in the CMR study protocol.
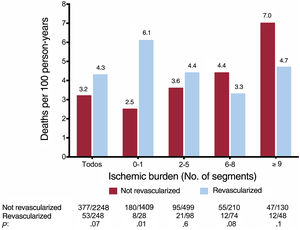
In the whole group, there was a nonsignificant trend to a higher annual mortality rate in patients who had received revascularization than those who had not; this association was significant in patients without ischemia or with mild ischemia (0-1 segments) (figure 3). In parallel to what was observed in the whole group (figure 1), in patients who had not undergone revascularization, the annual mortality rate increased as ischemic burden increased. However, there was a trend in the opposite direction in patients who had undergone revascularization (figure 3 and figure 4). Figure 2 illustrates the divergent effect of the extent of ischemic burden on all-cause mortality adjusted for whether or not they had undergone CMR-guided revascularization, both for the whole registry (Pinteraction=.003) and for the matched population (Pinteraction=.02).
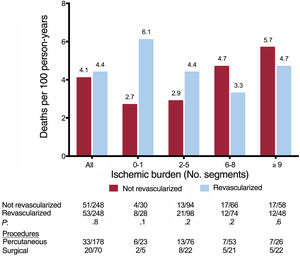
A dichotomous analysis was performed, dividing the patient sample into 2 categories according to their ischemic burden and analyzing the adjusted survival curves. Patients with nonextensive ischemic burden (≤ 5 segments) who had undergone revascularization showed a significantly higher risk of all-cause mortality than those who had not undergone CMR-guided revascularization in the whole registry, and there was a trend in the same direction in the matched patients. In those with extensive ischemia (> 5 segments), revascularization did not show statistically significant differences (figure 5).
Effect of stress CMR-guided revascularization on all-cause mortality: adjusted survival curves. On the adjusted survival curves, the patients with nonextensive ischemic burden (≤ 5 ischemic segments) who underwent CMR-guided revascularization showed a significantly higher risk of all-cause mortality than those who did not undergo CMR-guided revascularization in the whole registry (A) and a strong trend in the same direction in the matched patients (B). The cofactors that were independently association with all-cause mortality on multivariate analysis (age, male, DM, LVEF, LVEDV) and the propensity scoring in patients who underwent CMR-guided revascularization were used to adjust the survival curves for the whole registry (A and C). CMR, cardiac magnetic resonance; DM, diabetes mellitus; LVEDV, left ventricular end diastolic volume; LVEF, left ventricular ejection fraction.
The main finding in our study was that, in elderly patients with known or suspected CCS, a higher ischemic burden on vasodilator stress CMR was associated with a higher risk of all-cause mortality, demonstrating its value for risk stratification in this increasingly common situation. To our knowledge, this is the largest real-life registry that has been used to evaluate the prognostic value of stress CMR in patients older than 70 years.
Ischemic burden and risk stratificationPatients with CCS, especially those who have already had an acute event, have a high risk of recurrent ischemic events.18–20 In our cohort, which included elderly patients with CCS, there were 430 registered deaths from all causes during the long-term follow-up (> 4 deaths/100 person-years; 17.2% of the total). This indicates that it is a patient group with substantial risk, and it is therefore essential to perform risk stratification that allows treatment optimization in those with a worse prognosis. All-cause mortality was used as a single outcome variable because of its relevance and because it is the most verifiable clinical event with respect to guaranteeing the quality of retrospective data collection.
In the current recommenations,8 noninvasive imaging techniques, which include stress CMR, have become the first diagnostic step for patients with CCS. As expected, patients with a worse profile on CMR, that is, with greater volumes (eccentric remodeling), lower LVEF, and more extensive inducible ischemia and necrosis, had a higher mortality rate. These findings are consistent with previous studies by our group and other authors that show that the structural and functional parameters of stress CMR are strong prognostic predictors in the general population with CCS.9–11,13,21 Therefore, this technique, in addition to its recognized diagnostic role, could be of great clinical use for stratifying the risk of such important events as mortality.
Despite these points, there is little evidence on the use of noninvasive imaging techniques in the elderly population.12,22 Recently, an interesting pioneer study with a short series of 110 patients older than 70 years was published: Esteban-Fernández et al.12 observed that greater ischemia on stress CMR was associated with the composite outcome of death, acute coronary syndrome, and revascularization. Only 15 deaths were recorded (4 in the group with CMR positive for ischemia), and therefore the study did not have the sufficient statistical power to analyze the prediction of this important event. Given the size of the group, the authors did not carry out an analysis of the implications for decision-making. Our study demonstrates that, in a large group of patients older than 70 years, the risk of mortality from all causes increased in parallel with an increase in ischemic burden. This risk increased progressively from 13.1% in patients with 0-1 ischemic segments to 33.1% in those with more extensive ischemia (≥ 9 segments). This association was maintained after adjustment for strong independent predictors, the risk of death increasing by 4% [1%-7%] for each additional poststress ischemic myocardial segment (P=.006). However, the isolated value of a single CMR parameter in predicting a variable as serious as all-cause mortality was moderate, demonstrating the need for a complete evaluation of the patient as a whole.
After analysis of the annual mortality risk stratified by age and sex, our results indicated that, in an elderly population that poses a significant challenge for risk stratification, the ischemic burden derived from stress CMR was a good predictor of all-cause mortality, homogeneously and independently of sex, even in very advanced ages. All of this reinforces the idea that, while always taking into account the individual context of each patient, the current recommendations for the approach to CCS using noninvasive imaging techniques are perfectly applicable to the elderly population independently of sex and age group.
Ischemic burden and prognosis according to use of revascularizationRegarding the analysis of the effect of revascularization, our study was observational, therefore the findings must be considered merely exploratory. Two hundred and forty-eight CMR-guided revascularization procedures were registered, representing 9.9% of the total sample. There was a statistically nonsignificant trend to higher all-cause mortality in the group of patients who had undergone CMR-guided revascularization.
In the analysis performed according to the extent of ischemia, a differential prognostic impact was detected depending on whether or not an invasive strategy had been used. As also occurred in the whole group, in patients who had not undergone revascularization, the rate of events was progressively higher as a function of the number of ischemic segments. However, an opposite trend was observed in patients who had had revascularization: unlike patients who had not received CMR-guided revascularization, the benefit in terms of mortality reduction increased as the ischemic burden increased. Thus, ischemic burden had a divergent effect on risk of all-cause mortality depending on the treatment strategy used. This same trend was detected in a recent analysis of all patients included in the registry (not just elderly patients).13 The present study therefore confirms the validity of ischemic burden as determined on stress CMR to obtain information on the expected effect of revascularization in an elderly population.
In our study, the cut point where mortality risk inverted for patients who had and had not undergone revascularization was> 5 ischemic segments. In the group with nonextensive ischemia, those who underwent CMR-guided revascularization showed a significantly higher risk of death than those who did not receive revascularization. In contrast, in the group of patients with an extensive ischemic burden, no statistically significant differences were observed in risk of all-cause mortality as a function of coronary revascularization.
Despite the recent results of the ISCHEMIA trial,23 there is still ongoing debate on the treatment of patients with CCS. This debate about the improvement in prognosis derived from the addition of revascularization as standard to optimal medical treatment is even more pronounced in the case of elderly patients. This population is insufficiently represented in the trials used as the basis for clinical practice, and it is not uncommon for there to be a more conservative inertia in this group.1 Nonetheless, we must not forget that this is a heterogenous group, with a higher incidence of treatment-related complications, in whom is it essential to complete a holistic geriatric assessment and individualize the most appropriate treatment strategy in each case.24
Our results indicate that, both in the whole group and the matched population, there was an interaction between revascularization and subsequent mortality in elderly patients with CCS. There may even be a deleterious effect in patients without ischemia or with nonsevere ischemia; in contrast, at higher ischemic burdens, this negative effect disappeared and showed an opposite trend. However, when the group with extensive ischemic burden was analyzed separately, no net benefit was detected in revascularized patients.
The results of this section should be interpreted with caution, taking into account the observational nature and small sample size of the study, especially the study of subgroups based on extent of ischemic burden and the use of revascularization. Nonetheless, our findings support the idea that, in elderly patients with CCS, it is important to act cautiously when opting for an invasive approach, as there do not appear to be substantial survival benefits. In this situation, decision-making is a challenge and requires individualized assessment that prioritizes the patient's preferences and environment. Revascularization should be justified by a potential improvement in symptoms and quality of life, always bearing in mind the technical complexity, risk of complications, and clinical context of the geriatric population. The detection of a high ischemic burden on stress CMR, in addition to its clear diagnostic and prognostic usefulness, could be helpful together with the aforementioned factors for identifying which patients would have a more acceptable risk-benefit ratio from a coronary revascularization strategy.
Study limitationsSeveral aspects related to the study design and characteristics represent possible limitations to the interpretation of the results. First, in the current literature, there is no established threshold for defining a geriatric population, so it was decided to use 70 years in line with most of the literature reviewed. Likewise, our study does not take into account frailty criteria or other geriatric parameters that influence prognosis in this population. The limitations of the results obtained are those relating to nonrandomized observational registries. Our registry was designed to include a large number of patients over a long period of time. To avoid missing data and maximize the robustness of data collection, only a small number of clinical and CMR variables were defined in the database. Undoubtedly, the availability of additional data, such as those from coronary angiography or medical treatment, would allow many more collateral analyses to be performed that are currently not possible. All-cause mortality was chosen as a single outcome variable. It is the most verifiable and relevant clinical event, and the retrospective data review strategy within a unified electronic regional health system guaranteed the quality of the information obtained. A more complete view of patient outcomes could have been achieved by including other events such as the cause of death, symptom relief, unplanned revascularization procedures, and reinfarction.
CONCLUSIONSOur study indicates that ischemic burden as assessed on vasodilator stress CMR allows us to predict the risk of all-cause mortality in elderly patients with known or suspected CCS. Furthermore, this technique could be of use in decision-making, as it provides information that helps determine the best treatment strategy.
- -
There is a lack of evidence on the best treatment and prognostic stratification in elderly patients with CCS, due to their underrepresentation in large clinical trials.
- -
Although stress CMR is a very well-established technique in the clinical guidelines, due to limitations in its use there are no studies with a large population of elderly patients.
- -
Revascularization added to optimal medical treatment does not provide a prognostic benefit to all patients with CCS.
- -
Our study provides evidence on elderly patients with CCS studied with stress CMR in a large prospective patient cohort.
- -
As well as adding relevant prognostic information on all-cause mortality according to the magnitude of ischemic burden, it could help guide the treatment approach in this patient group.
This study has been jointly funded by the Instituto de Salud Carlos III, the Fondo Europeo de Desarrollo Regional (FEDER) and the Sociedad Española de Cardiología and Fundación Española del Corazón [References: PI17/01836, CIBERCV16/11/00486 and SEC/FEC-INV-CLI 21/024].
AUTHORS’ CONTRIBUTIONSA. Gabaldón-Pérez and C. Bonanad contributed equally to this study. A. Gabaldón-Pérez conceived the idea presented, collected data, wrote the article, and participated in the final review of the manuscript. C. Bonanad conceived the idea presented, collected data, wrote the article, and participated in the final review of the manuscript. S. García-Blas collected data, supervised the research, and participated in the writing and final review of the manuscript. J. Gavara designed and performed the statistical analysis, collected data, and participated in the final review of the manuscript. C. Ríos-Navarro collected data and participated in the final review of the manuscript. N. Pérez-Solé collected data and participated in the final review of the manuscript. E. de Dios collected data and participated in the final review of the manuscript. V. Marcos-Garcés collected data, collaborated in the writing of the article, and participated in the final review of the manuscript. H. Merenciano-González collected data and participated in the final review of the manuscript. J.V. Monmeneu collected data, collaborated in the writing of the article, and participated in the final review of the manuscript. M.P. López-Lereu collected data, collaborated in the writing of the article, and participated in the final review of the manuscript. J. Núñez supervised the research, obtained funding, validated the results of the research, and participated in the final review of the manuscript. F.J. Chorro supervised the research, obtained funding, validated the results of the research, and participated in the final review of the manuscript. V. Bodí conceived the idea presented, collected data, supervised and validated the final results of the research, obtained funding, and participated in the writing and final review of the manuscript.
CONFLICTS OF INTERESTNone declared.
Supplementary data associated with this article can be found in the online version available at https://doi.org/10.1016/j.rec.2021.08.004.