Microparticles are markers for cell activation and apoptosis and could provide valuable information that is not available from clinical data. This study assesses the clinical and biological relationship of small-sized microparticles in different forms of ischemic systolic heart failure and their relation to markers of inflammation and repair.
MethodsWe compared 49 patients with acute heart failure, 39 with stable heart failure and 25 patients with stable coronary artery disease. Small-size microparticles counts were determined by high-resolution flow cytometry. Moreover, 3 different monocyte subpopulations and their expression of inflammatory and adhesive scavenger receptors were analyzed using a conventional flow cytometer.
ResultsEndothelial CD144+ microparticle counts were decreased in heart failure groups (P=.008). Annexin V-binding microparticle counts were found increased in heart failure (P=.024) and in patients with lower functional class (P=.013). Platelet CD42b+ microparticle counts positively correlated with left ventricular ejection fraction (P=.006), and annexin V-binding microparticle counts with interleukin-6 levels in stable heart failure (P=.034). Annexin V-binding microparticle counts in the acute status strongly correlated with toll-like receptor-4 expression on all monocyte subsets (all P<.01). Three months after admission with acute heart failure, annexin V-binding microparticle counts were positively correlated with receptors for interleukin-6, CD163 and CD204 (all P<.05).
ConclusionsAnnexin V-binding microparticle counts constitute valuable hallmarks of acute decompensated state in systolic heart failure. The observed relationship between small-size annexin V-binding microparticles and scavenger receptors supports their involvement in the progression of the acute response to injury, and thus their contribution to the pathogenesis of acute decompensated heart failure.
Keywords
Systolic heart failure (HF) remains a debilitating and life-threatening condition despite considerable treatment achievements.1,2 Many major pathways for prothrombotic and proatherogenic changes and endothelial damage converge on an ischemic or hypertensive heart and the molecular processes represent a complex network of interacting pathways.3
Microparticles are valuable markers for cell activation and apoptosis.4 A previous study reported that endothelial microparticles levels reliably predict future cardiovascular events in patients with HF, suggesting that microparticles could be involved in the pathogenesis of several cardiovascular conditions apart from atherothrombotic complications.5 In addition, circulating microparticles are increased in subjects with cardiovascular risk factors and coronary artery disease (CAD),6,7 being recently related to indices of injury and repair in patients with acute coronary syndrome and independent predictors of future HF in non—ST-segment elevation myocardial infarction patients.8 Besides, the role of monocytes in ischemic conditions is uncertain. These functions include inflammatory response, regulation of thrombogenic status (eg, via tissue factor expression and modulation of fibrinolysis), but also beneficial properties related to scavenging of redundant/dangerous substances, angiogenesis, and repair.9,10 In view of the interrelationships between vascular dysfunction, inflammation, and apoptosis with cardiac function, it is conceivable that microparticles could potentially be a diagnostic marker related to pathophysiology of acute heart disease.11
In the present study, our objective was to investigate the relevance of microparticles in HF, using an approach that allows discrimination and quantification of a wide range of microparticles sized 0.1μm polystyrene beads or above (eg, “small-size” microparticles).12 This size range corresponds to the accepted definition of microparticles.13 We hypothesized that small-size microparticles generation and origin are related to distinct pathomechanisms assessed as monocytic activation indexes, and ultimately to the damage and severity of the disease. We therefore examined the relationship of circulating counts of small-size microparticles in patients with acute HF (AHF) and stable HF (SHF) of ischemic origin, who were compared to “disease control” patients with stable CAD and preserved left ventricular function. Additionally, we investigated relationships of small-size microparticles counts to expression of scavenger receptors on monocytes as markers of inflammation and repair to provide insight about the pathophysiological status of the disease.
METHODSStudy PopulationIn the following prospective study, we consecutively recruited 49 patients with AHF and 39 patients with SHF. The AHF group (all in New York Heart Association [NYHA] functional class IV) was defined in accordance with the European Society of Cardiology guidelines as the rapid onset/progression of HF symptoms and signs secondary to abnormal cardiac function requiring hospital admission.14 Patients with SHF were recruited from outpatient clinics (NYHA I-III); they presented with chronic HF with no deterioration in clinical condition. All patients with HF had left ventricular ejection fraction (LVEF) of ≤ 40% on echocardiography or left ventriculography. In order to evaluate the impact of HF, only patients with underlying CAD as the etiology of HF were recruited. Patients with acute coronary syndrome were excluded (chest pain with ST/T wave changes on electrocardiogram±positive troponin). Elapsed time between the last acute episode and hospitalization was > 6 months.
This would allow comparisons to be made with a suitable control group with CAD (n=25), preserved ventricular function, and no HF, but with a similar pattern of comorbidities, risk factors (eg, diabetes, hypertension) and background medication. “Disease controls” with stable CAD were defined as myocardial infarction > 6 months previously and/or angiographically documented stenosis > 50% in ≥ 1 coronary artery and LVEF ≥ 55 %. For all study groups, exclusion criteria included factors that could affect small-size microparticles counts and monocyte phenotype (infectious and inflammatory disorders, cancer, creatinine > 200μmol/L, steroids and hormone replacement therapy), atrial fibrillation, or moderate-severe valvular disease.
Blood Collection and Time-pointsFor laboratory analysis, non-fasting peripheral venous blood samples were collected from all participants and processed by flow cytometry within 60minutes for assessment of monocyte characteristics (fresh whole blood). Platelet-poor plasma was frozen and stored for consequent batched small-size microparticles and cytokine analysis (details below).
In order to assess the dynamics of small-size microparticles and monocyte parameters in AHF over time, blood samples were analyzed at the following time-points: a) during the first 24hours after admission; b) on the day of hospital discharge, and c) 3 months following hospital admission. A proportion of patients (n=14) did not complete follow-up due to study withdrawal (n=3) or death (n=11). The study was performed in accordance with the Declaration of Helsinki and was approved by the Warwickshire Research Ethics Committee. All participants provided written informed consent.
LaboratorySmall-size Microparticles AnalysisCitrated plasma was obtained after 15min centrifugation at 2800g at room temperature. Aliquots of the plasmas were frozen at –70°C for subsequent batched analysis of small-size microparticle/fluorescence-activated cell sorting array and underwent a single freeze-thaw cycle. The methodology for small-size microparticle analysis and appropriate controls has been described in previous publications.8,12,15 CD42b+ small-size platelet microparticles (sPMP), CD144+ small-size endothelial microparticles (sEMP), and small-size annexin V-binding microparticles (sAMP) were automatically quantified by the volumetric method of the flow cytometer (Apogee Flow Systems; Hertfordshire, United Kingdom). Intra-assay and inter-assay coefficients of variation were<5%. The limits of detection were<100 sMP/μL for sPMP and sEMP and<500 sMP/μL for sAMP.
Monocyte Activation AnalysisFlow cytometric analysis for distinct monocyte subsets was performed using fluorescence-activated cell sorting Calibur flow cytometer (Becton Dickinson; Oxford, United Kingdom). Monocyte subsets were defined as CD14++CD16–CCR2+ (Mon1, “classical”’, CD14++CD16+CCR2+ (Mon2, “intermediate”), and CD14+CD16++CCR2– (Mon3, nonclassical) using appropriate isotype controls and in keeping with current consensus.16,17 The anti-CD14, anti-CD16, and anti-CCR2 antibodies were mixed with 50μL EDTA (ethylenediaminetetraacetic acid) blood and phycoerythrin-conjugated antibodies (all from R&D Systems; Abingdon, United Kingdom) against toll-like receptor-4, CXCR4, vascular endothelium growth factor receptor 1 or CD34 (Becton Dickinson), and allophycocyanin-conjugated antibodies against interleukin (IL)-6, receptor, CD204, CD163, or vascular cell adhesion molecule-1 receptor. Expression of the surface markers was quantified as mean fluorescence intensity.18
Fluorescence-activated Cell Sorting Array AssaysPlasma levels of diverse interleukins (IL-6, IL-10, IL-1β) and monocyte chemoattractant protein-1 were measured by cytometric bead array technology with commercially available reagents sets (all from BD Biosciences; Oxford, United Kingdom), according to the manufacturer's recommendations.19 The limits of detection for ILs and monocyte chemoattractant protein-1 were<0.1 ng/mL and<5 ng/mL, the intra- and inter-assay coefficients of variation for all assays were<8%. The fluorescence-activated cell sorting Calibur flow cytometer was used for data acquisition, with flow cytometric analysis program Array v2.0.2 software (Burnsville, Minnesota, United States) for data analysis.
Hematological and Biochemical AnalysisHematological analysis was performed in our department using automated cell blood counter, ADVIATM 120 (Bayer, Germany), as per standard techniques. All analyses were performed following blood extraction.
Statistical AnalysisData are expressed as mean (SD, standard deviation) for normally distributed data or median and interquartile range for non-normally distributed data. Cross-sectional data were analyzed by one-way analysis of variance and follow-up data were analyzed by repeated measures ANOVA (Sidak confidence interval adjustment). The Kruskal-Wallis or Friedman test was used for non-normally distributed data. A post-hoc Tukey test was performed to assess inter-group differences, where appropriate. Logarithmic transformation of non-normally distributed variables was performed when possible, prior to post-hoc analysis of ANOVA. Mann-Whitney test was used to compare small-size microparticles counts between patients with stable CAD and 3-month values in AHF patients. Correlations between study parameters at admission with HF were assessed using Pearson method (for normally distributed parameters) or Spearman method (for non-normally distributed parameters). Multivariate analysis by linear regression was used to identify the factors associated to sAMP counts in severe dyspnea patients. Variables with p ≤ 0.15 in the univariate analysis were included into the multivariate regression model. Significance level was considered P<.05 and omnibus P-value ≤ .017 (for Mann-Whitney and Wilcoxon tests). SPSS 18.0 software was used for statistical analyses (SPSS Inc.; Chicago, Illinois, United States).
RESULTSPatient Clinical CharacteristicsThe study groups were comparable for age, sex, ethnicity, blood pressure, body mass index, hypertension, diabetes, and smoking (Table 1). Beta-blockers were less often used in patients admitted with AHF (P<.001) but no differences were found for other prescribed treatments (omnibus P>.017).
Baseline Characteristics of the Studied Populations
| AHF (n=49) | SHF (n=39) | Stable CAD (n=25) | P value (ANOVA or Kruskal-Wallis) | P value AHF vs CAD | P value SHF vs CAD | P value AHF vs SHF | |
|---|---|---|---|---|---|---|---|
| Age, mean (SD), years | 66 (12) | 70 (8) | 66 (7) | .12 | |||
| Male, % | 36 (72) | 32 (82) | 19 (76) | .55 | |||
| Ethnicity, % Caucasians | 44 (88) | 34 (88) | 20 (80) | .62 | |||
| Systolic blood pressure, mean (SD), mmHg | 137.9 (27.5) | 124.6 (24.3) | 127.3 (15.8) | .15 | |||
| Diastolic blood pressure, mean (SD), mmHg | 79.6 (16.9) | 73.8 (11.2) | 71.5 (10.7) | .18 | |||
| Body mass index, mean (SD), kg/m2 | 28.4 (3.8) | 28.1 (4.6) | 27.5 (3.4) | .91 | |||
| Hypertension, % | 29 (58) | 18 (46) | 14 (56) | .56 | |||
| Diabetes, % | 20 (40) | 8 (21) | 5 (20) | .08 | |||
| Smoking, % | 25 (50) | 22 (56) | 9 (36) | .35 | |||
| Hemoglobin, mean (SD) | 12.2 (1.9) | 13.9 (1.7) | 13.9 (1.6) | < .001 | .001 | .98 | .002 |
| White blood cells, mean (SD), 103/μL | 8.0 (2.5) | 6.8 (1.5) | 5.9 (0.8) | .001 | .02 | .21 | < .001 |
| Neutrophil/lymphocyte ratio, mean (SD) | 6.1 (4.1) | 3.0 (1.80) | 2.4 (4.13) | < .001 | < .001 | .77 | < .001 |
| Platelet count (103/μL) | 254.5 (91.7) | 227.9 (51.6) | 233.1 (62.9) | .31 | |||
| Mean platelet volumen, mean (SD) (fL) | 7.8 (0.9) | 7.8 (0.8) | — | .95 | |||
| LVEF, % | 25.0 [22.5-40.0] | 34.5 [21.0-35.0] | 55.0 [55.0-62.5] | < .001 | < .001 | < .001 | .98 |
| NYHA I | 0 (0) | 12 (31) | — | ||||
| NYHA II | 0 (0) | 23 (59) | — | ||||
| NYHA III | 0 (0) | 4 (10) | — | ||||
| NYHA IV | 49 (100) | 0 (0) | — | ||||
| Death during hospitalization, % | 2 (4) | — | — | ||||
| Death after discharge, % | 9 (18) | — | — | ||||
| Angina, % | 18 (36) | 6 (15) | 13 (52) | .004 | .28 | .004 | .07 |
| Previous myocardial infarction, % | 28 (56) | 27 (69) | 12 (48) | .27 | |||
| Previous percutaneous intervention, % | 6 (12) | 10 (26) | 15 (60) | < .001 | < .001 | .002 | .27 |
| Previous coronary artery bypass graft, % | 7 (14) | 8 (21) | 10 (40) | .025 | .02 | .12 | .73 |
| Previous cerebrovascular accident, % | 5 (10) | 7 (18) | 3 (12) | .55 | |||
| Previous chronic obstructive pulmonary disease, % | 7 (14) | 2 (5) | 1 (4) | .23 | |||
| Creatinine, mean (SD), μmol/L | 131.7 (44.2) | 104.1 (24.4) | 86.9 (17.4) | < .001 | < .001 | .12 | .001 |
| Acetylsalicylic acid, % | 36 (72) | 31 (80) | 22 (88) | .15 | |||
| Thienopyridines, % | 20 (40) | 10 (26) | 11 (44) | .21 | |||
| Statin, % | 42 (84) | 33 (85) | 22 (88) | .66 | |||
| ACE inhibitor,[% | 39 (78) | 33 (85) | 17 (68) | .30 | |||
| Thiazide, % | 3 (6) | 3 (8) | 5 (20) | .12 | |||
| Beta-blocker, % | 19 (38) | 29 (75) | 19 (76) | < .001 | .001 | .91 | .001 |
| Calcium channel blocker, % | 9 (18) | 3 (8) | 8 (32) | .034 | .23 | .026 | .41 |
| Loop diuretic, % | 49 (100) | 33 (85) | — | .019 | — | — | .019 |
| Warfarin, % | 9 (18) | 13 (33) | 4 (16) | .25 | |||
| Brain natriuretic peptide-1, pg/mL | 547 [239-1334] | 71.0 [34-256] | — | < .001 | — | — | < .001 |
| Interleukin-6, ng/mL | 9.0 [5.8-17.1] | 3.0 [1.5-4.6] | 3.1 [1.9-5.4] | < .001 | < .001 | .70 | < .001 |
| Interleukin-1β, ng/mL | — | — | 0.6 [0.2-0.8] | — | — | — | — |
| Interleukin-10, ng/mL | — | — | 1.1 [0.0-1.5] | — | — | — | — |
| Monocyte chemoattractant protein-1, mean (SD), ng/mL | 95.7 (64.8) | 132.9 (89.9) | 64.8 (48.9) | .003 | .012 | .003 | .65 |
| sPMP (103/μL) | 13.0 [2.8-42.9] | 9.9 [3.2-22.7] | 15.2 [5.3-35.6] | .011 | .14 | .026 | .58 |
| sEMP (103/μL) | 5.9 [2.1-13.0] | 3.2 [1.1-10.7] | 15.0 [3.6-35.9] | .008 | .062 | .004 | .056 |
| sAMP (103/μL) | 82.7 [54.6-123.2] | 64.0 [53.0-80.1] | 50.6 [32.0-78.4] | .024 | .017 | .09 | .11 |
ACE inhibitor, angiotensin-converting enzyme inhibitor; AHF, acute heart failure; ANOVA, analysis of variance; CAD, coronary artery disease; LVEF, left ventricular ejection fraction; NYHA: New York Heart Association; sAMP, small-size annexin V-binding microparticles; SD, standard deviation; sEMP, small-size endothelial microparticles; SHF, stable heart failure; sPMP, small-size platelet microparticles;.
Data are expressed as No. (%), mean (standard deviation) or median [interquartile range] for normally and non-normally distributed variables.
Pairwise comparisons (Tukey Post-hoc or Mann-Whitney test) were significant for omnibus P-value is ≤ .017.
Hematological parameters for acute patients at admission were higher than those for the control group or SHF (Table 1) except for hemoglobin levels (P<.001). Clinically stable CAD and SHF patients had lower neutrophil/lymphocyte ratio (P<.001) and leukocyte counts (P=.001). Only platelet count and mean platelet volume remained unaltered, irrespectively of the patient group and treatment prescribed.
In the present study, levels of IL-6 and brain natriuretic peptide-1 in AHF patients were significantly higher than in CAD or SHF patients (P=.001). Macrophage chemoattractant protein-1 rose in both AHF (P=.012) and SHF (p=.003) compared to CAD patients, although there was no significant difference between the two disease subgroups (Table 1). Creatinine levels were higher in AHF than in SHF and CAD (P<.001 and P=.001, respectively). Clinical status of patients with AHF at admission (100% NYHA IV) was followed up 3 months after acute decompensation: 6% NYHA I, 30% NYHA II, 24% NYHA III, and 12% NYHA IV. Acute-phase cytokines and hematological parameters were also followed up (data not shown).
Circulating Small-size Microparticle Counts in Cross-sectional and Longitudinal StudiesAt the baseline time-point of AHF, small-size microparticle counts were not significantly different from counts in the SHF group (Figure 1). The SHF patients had lower sEMP counts compared to the CAD group (P=.004); sPMP count differences (P=.026) were not significant according to the omnibus P-value (Figure 1). Patients with AHF at admission had significantly higher sAMP counts (P=.017).
Small-size microparticle counts (103 sMP/μL) in platelet-poor plasma in the cross-sectional study. Comparison of small-size microparticle counts from stable coronary artery disease (n=25) patients vs acute heart failure during the first 24h after admission (day 1, n=49) and stable heart failure (n=39) (Mann-Whitney and Kruskal-Wallis tests). A: platelet CD42b+ sMP. B: endothelial CD144+ sMP. C: annexin V binding+ sMP. AHF, acute heart failure; CAD, coronary artery disease; sAMP, small-size annexin V-binding microparticles; sEMP, small-size endothelial microparticles; SHF, stable heart failure; sPMP, small-size platelet microparticles. The parallel lines indicate median value and range [interquartile range].
As illustrated in Figure 2, no significant changes in sPMP counts were shown over the 3 months of acute decompensation of HF (P=.048), although sEMP counts significantly increased over the 3 months (P=.014). No differences were found between small-size microparticle counts of different origin for AHF and CAD patients at 3 months after admission (Figure 2). In a cross-sectional study comparing SHF and AHF after three months of admission, higher sEMP counts were found in acute patients (P=.008) (data not shown).
Dynamics of small-size microparticle counts (103 sMP/μL) following acute heart failure over the following 3 months of acute decompensation (n=32) (Friedman test). A: platelet CD42b+ sMP. B: endothelial CD144+ sMP. C: annexin V binding+ sMP. AHF, acute heart failure; CAD, coronary artery disease; sAMP, small-size annexin V-binding microparticles; sEMP, small-size endothelial microparticles; sPMP, small-size platelet microparticles. Comparison of small-size microparticle counts between the first 24 h after admission [AHF_1], day of hospital discharge, [AHF_2], 3 months after admission [AHF_3], and disease group (coronary artery disease) were also performed (Mann-Whitney test). The parallel lines indicate median value and range [interquartile range].
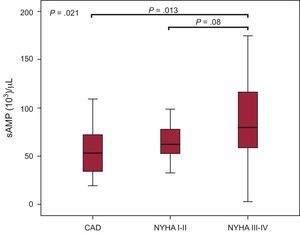
Of hematological parameters, only hemoglobin levels were correlated with small-size microparticle counts in the different groups and over time. In fact, sAMP counts positively correlated with hemoglobin in AHF at admission (r=0.41; P=.026) and after 3 months, they also increased with sPMP and sEMP counts (r=0.49; P=.022 and r=0.43; P=.050, respectively). Left ventricular ejection fraction at admission with AHF was positively correlated with sPMP counts (r=0.42; P=.006) and patients with LVEF<25% presented lower sPMP counts (P=.013). This relationship was lost after 3 months of admission. When all HF patients were considered, higher sAMP counts were also associated with severe functional class (NYHA III-IV) compared to CAD (P=.013) (Figure 3). Concerning drug treatments, lower sPMP counts were associated with the use of beta-blockers (β=–0.38; P=.016), while aspirin use was related to lower sAMP counts (β=–0.42; P=.008) in SHF patients (data not shown).
Comparison of small-size annexin V-binding microparticle counts (103 sMP/μL) in coronary artery disease and heart failure patients. CAD, coronary artery disease; NYHA, New York Heart Association; sAMP, small-size annexin V-binding microparticles. Functional status of heart failure patients was assessed with New York Heart Association class (Mann-Whitney and Kruskal-Wallis tests). Box plots indicate median and range [interquartile range].
Only IL-6 was positively correlated with sAMP counts in SHF (r=0.35; P=.034). In SHF, creatinine was also positively correlated with the different small-size microparticle origins (all P<.05) (data not shown).
Monocyte Scavenger Receptors Expression and Small-size Annexin V-binding Microparticle CountsSince sAMP have been described as a marker of apoptosis,20 we assessed the relationship between sAMP counts and different scavenger receptors of monocyte subsets in the AHF longitudinal study (Table 2). Toll-like receptor-4 expression on the 3 subsets of monocyte positively correlated with sAMP counts at admission with AHF (Mon1, r = 0.38; P = .009; Mon2, r = 0.42; P = .004; Mon3, r = 0.43; P=.003). This relationship was maintained on the day of discharge (AHF_2) but was lost at 3 months after admission (AHF_3). On the day of discharge, vascular cell adhesion molecule-1 was negatively correlated with sAMP counts (Mon1, r = –0.43; P=.005; Mon2, r = –0.57; P<.001; Mon3, r = –0.42; P=.007) and remained after 3 months except for Mon3 subpopulation. Interleukin-6 receptor, CD163, and CD204 were not correlated with sAMP at time-points 1 or 2, but a relationship appeared with the recovery of a more favorable clinical condition.
Correlations Between Small-size Annexin V-binding Microparticles Counts and Expression of Surface Receptors in the Different Monocyte Subpopulations and Time-points
| AHF_1a (n=49) | AHF_2b (n=46) | AHF_3c (n=35) | ||||
|---|---|---|---|---|---|---|
| r | P value | r | P value | r | P value | |
| Toll-like receptor-4 expression | ||||||
| Mon1 | 0.38 | .009 | 0.40 | .012 | 0.30 | .10 |
| Mon2 | 0.42 | .004 | 0.39 | .013 | 0.31 | .09 |
| Mon3 | 0.43 | .003 | 0.46 | .003 | 0.42 | .015 |
| Vascular cell adhesion molecule-1 expression | ||||||
| Mon1 | –0.29 | .059 | –0.43 | .005 | –0.54 | .002 |
| Mon2 | –0.18 | .23 | –0.57 | <.001 | –0.49 | .005 |
| Mon3 | 0.03 | .87 | –0.42 | .007 | –0.21 | .14 |
| Interleukin-6 receptor expression | ||||||
| Mon1 | –0.13 | .40 | 0.07 | .68 | 0.39 | .03 |
| Mon2 | –0.08 | .61 | –0.04 | .81 | 0.44 | .012 |
| Mon3 | 0.09 | .57 | 0.1 | .56 | 0.13 | .47 |
| CD163 expression | ||||||
| Mon1 | 0.06 | .69 | 0.12 | .49 | 0.38 | .032 |
| Mon2 | –0.09 | .56 | –0.09 | .59 | 0.36 | .046 |
| Mon3 | 0.18 | .24 | –0.15 | .36 | 0.37 | .035 |
| CD204 expression | ||||||
| Mon1 | 0.24 | .11 | 0.42 | .008 | 0.44 | .012 |
| Mon2 | 0.27 | .065 | 0.25 | .13 | 0.46 | .009 |
| Mon3 | 0.30 | .09 | 0.10 | .55 | 0.27 | .133 |
AHF, acute heart failure.
Expression of the surface markers is shown as mean fluorescence intensity.
Calculations were based on Spearman correlation analysis.
In order to identify independent determinants of plasma sAMP counts, a bivariate test was initially performed in severe dyspnea patients (NYHA IV). The objective for the single parameter analysis was to screen variables and to exclude those which had no association with sAMP counts (P ≤ .15). Bivariate analysis showed that sAMP counts were more likely related to ethnicity, lower LVEF, acetylsalicylic acid, and thienopyridines. Table 3 shows the results of multivariate analysis model (P ≤ .005), South Asian ethnicity, and thienopyridine treatment (all P<.05) significantly influence sAMP counts. Biomarkers of ventricular remodeling (LVEF and brain natriuretic peptide-1) do not seem to contribute to sAMP counts in the multivariate study.
Multivariate Analysis for the Variables of Small-size Annexin V-binding Microparticle Counts in Acute Heart Failure Patients With Severe Dyspnea (New York Heart Association functional class IV)*
| Factors R2=0.38 | Unstandardized coefficients | Standardized coefficients | t | P value | |
|---|---|---|---|---|---|
| B | Standard deviation | β | |||
| (Constant) | –19793.77 | 32413.21 | –0.61 | .55 | |
| Ethnics | 59430.42 | 22918.52 | 0.36 | 2.59 | .014 |
| LVEF | 1637.70 | 891.99 | –0.26 | 1.84 | .074 |
| Acetylsalicylic acid | 17719.16 | 18801.84 | 0.14 | 0.94 | .35 |
| Thienopyridines | 35478.15 | 16509.48 | –0.30 | –2.15 | .038 |
| Diabetes | 1264.86 | 16738.20 | 0.01 | 0.08 | .94 |
B: regression coefficient. LVEF, left ventricular ejection fraction.
Ethnic refers to Caucasians vs South Asians (88% Caucasians).
Not transformed small-size annexin V-binding microparticles values were used in the analysis.
This is the first study showing increased circulating sAMP counts following AHF decompensation, compared with control subjects of similar etiology. Higher sAMP counts were associated with severe functional status (NYHA III-IV) and parallel with monocytic markers of repair and inflammation. Secondly, our study shows how a quantitative evaluation of biomarkers from pathological processes such as apoptosis (sAMP), platelet activation (sPMP), and monocyte receptors (leading to atherothrombogenic complications) can be clinically useful and valuable for the diagnosis of high-risk HF patients with an ischemic etiology.
Microparticle analysis is not yet used for clinical purposes, largely due to existing limitations of available methods for microparticle quantification and blood sample handling.21 Utilization of flow cytometers specifically designed for analysis of small-size particles is likely to provide considerable methodological advantages and should be the preferable option. Our group recently compared smaller vesicles termed “small-size microparticles” in patients with CAD and acute coronary syndromes, showing lower sEMP counts in acute ischemia.8 In the current data, it is unclear why sPMP and sEMP counts decrease with HF while sAMP counts increase, which suggests that sAMP could originate from different tissues or cells and display distinct functional sensitivity to ischemia. A recent study shows that increased counts of microparticles after cardiac stress seem to be a normal physiological response that is diminished in a worse clinical status, which could explain such lower counts in AHF and SHF.22 It is also plausible that the treatment causes impairment of small-size microparticle release, with beta-blockers already shown to inhibit monocyte and platelet microparticle release in hypertensive patients.23,24 In accordance, the use of thienopyridines significantly influences a decrease of sAMP release in advanced functional class (NYHA IV).
Recent studies have evaluated the association between circulating microparticle counts and several cardiovascular pathologies, denoting a link with severity of the disease and even suggesting it as a new prognostic biomarker in HF.5 In order to quantify sAMP utility for risk stratification, we divided patients into 2 groups depending on clinical manifestations and LVEF (but presenting similar comorbidities). We also analyzed other biomarkers corresponding to pathophysiological pathways (ventricular remodeling, inflammation, monocyte activation, and routinely-obtained laboratory parameters). Moreover, reduced renal function is increasingly common in patients with HF and is a well-documented cause of anemia.25 Lower sPMP counts in AHF are here associated with lower LVEF, suggesting that the release of small-size microparticles could be related to the degree of heart ischemic insult. Correlations between platelet microparticle counts and ejection fraction were also evident in patients with ST-segment elevation myocardial infarction,7 although microparticle gate was defined using 0.5 μm to 1.5 μm beads. Besides, small-size microparticle counts were also compared with the severity of the disease, resulting in a positive association between worse status (NYHA III-IV) and higher sAMP counts. The present study thus supports that sAMP (at admission) and sEMP counts (3 months postadmission) could represent useful biomarkers to discriminate the pathological status of patients with acute decompensated HF of ischemic etiology. However, it is important to point out that the clinical usefulness of microparticle counts as markers of worse clinical condition in a HF population is at present unclear and experimental studies are needed to prove that sAMP are not merely a biomarker but are involved in the pathogenesis of HF and CAD.
Research interest in monocyte heterogeneity and scavenger receptors has gained strong thrust in the past decade.16–19,26 Our previous aim was to investigate whether levels of monocyte scavenger receptors differed between acute and chronic HF patients;27 however, more investigations were needed to determine the pathophysiological relationship with other circulating elements, such as small-size microparticles. Among the antibodies with different specificities used in this study, Toll-like receptor-4, which is related to atherosclerotic plaque instability,28 was found to increase in parallel with sAMP counts, and then disappeared regularly after discharge. After improvement in patient symptoms, sAMP counts decreased slowly while VCAM-1 expression increased, suggesting a reinitiating adhesion after the acute phase.29 The IL-6 receptor expression and sAMP counts decreased in parallel after 3 months of acutely decompensated HF, which is in agreement with raised IL-6 levels shown in AHF to interrupt the antibody labeling. IL-6 induces the production of acute phase proteins, including C-reactive protein by liver cells.30 CD204 fluorescence intensity was not correlated with sAMP counts in AHF at admission (higher expression and thus clearance of apoptotic elements) but it positively appeared after discharge. Being involved in the clearance of apoptotic cells,31 it is tempting to speculate that CD204 also could be involved in the clearance of sAMP, but this mechanism is avoided or overflowed in acute states. Toll-like receptor-4, VCAM-1, IL-6 receptor, CD163, and CD204 are critical in leukocyte activation, clearance, and accumulation, being related to vascular function and inflammation. Our present study now points out that sAMP counts are representative of a pro-atherogenic condition by occurring together with monocyte expression of adhesion molecules. Nonetheless, the observational nature of the study does not allow us to determine causality and specific roles in the acute phase of HF.
LimitationsThe study is descriptive in its nature, as it compares SHF patients and patients with more severe HF (AHF), which is not representative of the entire population but is the focus of most clinical trials on HF. The study design prevents comprehensive equilibration of the tested groups regarding all clinical and demographic variables, and this leaves a possibility of a related bias. Our study is also limited by the relatively small numbers of patients. The pathophysiological significance of small-size microparticles in HF and their prognostic value would need to be fully established. Notwithstanding interpretative limitations of plasma biomarkers, the present study has found correlations between sAMP counts and various inflammation and repair biomarkers, as well as association with features of HF disease severity.
CONCLUSIONSPatients with HF have increased sAMP counts as an index of acute damage and lesser functional capacity. The relationship between sAMP and scavenger receptors expression may result in a reduced functional potential for repair, which could contribute to the pathogenesis of acute decompensated HF. Whether small-size microparticles play a role as messengers of biological information and direct regulators of pathophysiological processes and contribute to the causal progression of the disease remains to be established.
FUNDINGThe research leading to these results has received funding from Heart Research UK [RG2579/09/10], European Society of Cardiology, and the People Programme (Marie Curie Actions) of the European Union's Seventh Framework Programme (FP7/2007-2013) under REA grant agreement n° 608765.
CONFLICTS OF INTERESTNone declared.
S. Montoro-García holds postdoctoral position at the UCAM (Universidad Católica San Antonio de Murcia), Murcia, Spain.



![Small-size microparticle counts (103 sMP/μL) in platelet-poor plasma in the cross-sectional study. Comparison of small-size microparticle counts from stable coronary artery disease (n=25) patients vs acute heart failure during the first 24h after admission (day 1, n=49) and stable heart failure (n=39) (Mann-Whitney and Kruskal-Wallis tests). A: platelet CD42b+ sMP. B: endothelial CD144+ sMP. C: annexin V binding+ sMP. AHF, acute heart failure; CAD, coronary artery disease; sAMP, small-size annexin V-binding microparticles; sEMP, small-size endothelial microparticles; SHF, stable heart failure; sPMP, small-size platelet microparticles. The parallel lines indicate median value and range [interquartile range]. Small-size microparticle counts (103 sMP/μL) in platelet-poor plasma in the cross-sectional study. Comparison of small-size microparticle counts from stable coronary artery disease (n=25) patients vs acute heart failure during the first 24h after admission (day 1, n=49) and stable heart failure (n=39) (Mann-Whitney and Kruskal-Wallis tests). A: platelet CD42b+ sMP. B: endothelial CD144+ sMP. C: annexin V binding+ sMP. AHF, acute heart failure; CAD, coronary artery disease; sAMP, small-size annexin V-binding microparticles; sEMP, small-size endothelial microparticles; SHF, stable heart failure; sPMP, small-size platelet microparticles. The parallel lines indicate median value and range [interquartile range].](https://static.elsevier.es/multimedia/18855857/0000006800000011/v1_201511051439/S1885585715000420/v1_201511051439/en/main.assets/thumbnail/gr1.jpeg?xkr=eyJpdiI6InJ4azVxYjJJZ3dKQ1dlTE0yMGJCK3c9PSIsInZhbHVlIjoienBYd0VVTURUdXFSVm1xRGQ0TlRuaXlpRGlRTVJBZ1FJdFlISWJnSDV2UT0iLCJtYWMiOiJhNzBkNjUxYjAwYTRmNDJjMzUyNDNjYzkwYzM5YzFjMDBjYWQyZjRhMDQ0Njk2OTZjYWMyYzY4N2QxNzkzNDI3IiwidGFnIjoiIn0=)
![Dynamics of small-size microparticle counts (103 sMP/μL) following acute heart failure over the following 3 months of acute decompensation (n=32) (Friedman test). A: platelet CD42b+ sMP. B: endothelial CD144+ sMP. C: annexin V binding+ sMP. AHF, acute heart failure; CAD, coronary artery disease; sAMP, small-size annexin V-binding microparticles; sEMP, small-size endothelial microparticles; sPMP, small-size platelet microparticles. Comparison of small-size microparticle counts between the first 24 h after admission [AHF_1], day of hospital discharge, [AHF_2], 3 months after admission [AHF_3], and disease group (coronary artery disease) were also performed (Mann-Whitney test). The parallel lines indicate median value and range [interquartile range]. Dynamics of small-size microparticle counts (103 sMP/μL) following acute heart failure over the following 3 months of acute decompensation (n=32) (Friedman test). A: platelet CD42b+ sMP. B: endothelial CD144+ sMP. C: annexin V binding+ sMP. AHF, acute heart failure; CAD, coronary artery disease; sAMP, small-size annexin V-binding microparticles; sEMP, small-size endothelial microparticles; sPMP, small-size platelet microparticles. Comparison of small-size microparticle counts between the first 24 h after admission [AHF_1], day of hospital discharge, [AHF_2], 3 months after admission [AHF_3], and disease group (coronary artery disease) were also performed (Mann-Whitney test). The parallel lines indicate median value and range [interquartile range].](https://static.elsevier.es/multimedia/18855857/0000006800000011/v1_201511051439/S1885585715000420/v1_201511051439/en/main.assets/thumbnail/gr2.jpeg?xkr=eyJpdiI6Imh4cnlXQ0ZjbVpvNUJZS2w4TzFkRkE9PSIsInZhbHVlIjoiVHlReUFoWnY5WFJZVTBaT0pPWDZWMWJySXo0cEJCcXhDS2pENUhLZ21EWT0iLCJtYWMiOiI4NjE1YWI4MmNiYmNiNmIxY2Q0YzcwZTNiN2QxNGM5NTYxMjk1MDM2MTJhNGQ4YTFkZjZmOTU0NGRhZWM1NTE3IiwidGFnIjoiIn0=)
![Comparison of small-size annexin V-binding microparticle counts (103 sMP/μL) in coronary artery disease and heart failure patients. CAD, coronary artery disease; NYHA, New York Heart Association; sAMP, small-size annexin V-binding microparticles. Functional status of heart failure patients was assessed with New York Heart Association class (Mann-Whitney and Kruskal-Wallis tests). Box plots indicate median and range [interquartile range]. Comparison of small-size annexin V-binding microparticle counts (103 sMP/μL) in coronary artery disease and heart failure patients. CAD, coronary artery disease; NYHA, New York Heart Association; sAMP, small-size annexin V-binding microparticles. Functional status of heart failure patients was assessed with New York Heart Association class (Mann-Whitney and Kruskal-Wallis tests). Box plots indicate median and range [interquartile range].](https://static.elsevier.es/multimedia/18855857/0000006800000011/v1_201511051439/S1885585715000420/v1_201511051439/en/main.assets/thumbnail/gr3.jpeg?xkr=eyJpdiI6IklnOGQyUTY2eEt0OFZCS3h1SW1JeGc9PSIsInZhbHVlIjoiYWkzRVNocGY5ZUora3RIcnptUXVIMnhvVkJSUTJYY2RUZmJqakxZZTBvWT0iLCJtYWMiOiI2OTI2NWEzYzQwNmQ0MjEyOWZiMmI0MDczNTQxYWZlY2NjNzUwMzgyNDU1OGM0MDMwMmM5ZDVlZDhjNzUxZDAxIiwidGFnIjoiIn0=)