The aim of the present study was to examine the prognostic significance of heart rate and its trend in heart transplantation.
MethodsThis observational study enrolled 170 patients who received a bicaval heart transplant between 1995 and 2005; all were in sinus rhythm. The resting heart rate was determined via electrocardiography at the end of the first posttransplant year and annually until the tenth year. Cox analysis was used to evaluate the incidence of adverse events with a mean (standard deviation) follow-up of 8.9 (3.1) years. The primary study end point was the composite outcome of death or graft dysfunction.
ResultsThe resting heart rate at the end of the first posttransplant year was an independent predictor of the primary composite end point (hazard ratio=1.054; 95% confidence interval, 1.028-1.080; P<.001) and was significantly associated with total mortality (hazard ratio=1.058; 95% confidence interval, 1.030-1.087; P<.001) and mortality from cardiac causes (hazard ratio=1.069; 95% confidence interval, 1.026-1.113; P=.001), but not with graft dysfunction (hazard ratio=1.028; 95% confidence interval, 0.989-1.069; P=.161). For patients with a heart rate ≥ 105 or<90 bpm vs those with 90-104 bpm, the hazard ratios of the primary end point were 2.233 (95% confidence interval, 1.250-3.989; P=.007) and 0.380 (95% confidence interval, 0.161-0.895; P=.027), respectively. Heart rate tended to decrease in the first 10 years after transplantation (P=.001). Patients with a net increase in heart rate during follow-up showed a higher incidence of adverse events.
ConclusionsAn elevated heart rate is an adverse prognostic marker after heart transplantation.
Keywords
An elevated heart rate (HRT) is an independent marker of cardiovascular risk.1 Heart rate is strongly associated with the incidence of cardiovascular events in healthy individuals2 and patients with hypertension,3 coronary disease,4 and heart failure (HF).5 In addition, chronic treatment with heart rate-reducing agents, such as beta-blockers and ivabradine, improves prognosis in certain subgroups of patients with heart disease.6–8
Heart transplantation (HTx) continues to be the alternative therapy of choice in patients with refractory HF. For carefully selected candidates, HTx offers excellent long-term survival and quality of life.9,10 As a result of autonomic denervation, HTx patients have a higher resting HRTthan individuals with native hearts.11 Although this finding is often considered normal, some studies have indicated that HTx recipients with a higher heart rate may have worse survival.12–14 In one of these studies,14 the reduced survival was attributed to higher mortality from graft vascular disease (GVD). However, other authors12 failed to see differences in the distribution of causes of death according to HRT values. Thus, the causal association between heart rate and GVD is controversial.15–17
The aim of the present study was to analyze the prognostic significance of HRT in HTx patients, particularly focusing on its association with survival, causes of death, and graft function, as well as to describe its long-term trend.
METHODSStudy PopulationA retrospective analysis was conducted of a historical cohort of adult patients (> 18 years) who received an orthotopic HTx in our center between 1995 and 2005. The study included all patients who underwent surgery using a bicaval technique and who survived at least 1 year and were at that time in sinus rhythm. The following patients were excluded: those with repeat HTx, multiorgan transplantation, severe anemia (hemoglobin<10 g/dL), a pacemaker, or treatment with beta-blockers, diltiazem, verapamil, digoxin, amiodarone, or ivabradine. The study protocol was approved by the Comité Autonómico de Ética en la Investigación de Galicia.
ProtocolPatients were treated according to the local protocol. All patients received induction therapy with muromonab-CD3 or basiliximab during the immediate postoperative period. The maintenance immunosuppressive regimen comprised various combinations of prednisone, calcineurin inhibitors (tacrolimus or cyclosporine), antiproliferative agents (mycophenolate mofetil or azathioprine), and mTOR inhibitors (everolimus or sirolimus).
Endomyocardial biopsies were systematically performed during the first post-HTx year and thereafter if there was suspicion of acute rejection. Antidonor antibodies and immunopathological markers of humoral rejection were also measured if there were suggestive clinical findings. Coronary angiography was initially performed only in patients suspected of having GVD. However, from 2003 onward, this procedure was also performed after 1 month and 1, 5, and 10 years after the HTx in asymptomatic patients, unless contraindicated.
VariablesStudy data were retrospectively collected via medical history review. The resting HRT was determined from resting electrocardiograms performed in stable patients during regular visits to the outpatient service. Baseline HRT was determined at the end of the first post-HTx year and thereafter at annual intervals until the tenth post-HTx year. The baseline determination was calculated as the mean of all HRT measurements made via electrocardiograms obtained in outpatient visits between the tenth and twelfth month (fourth trimester) after the HTx. Updated information on the vital status of all study patients was obtained in March 2012. No patients were lost to follow-up.
The primary end point of the study was the composite outcome of all-cause mortality or graft dysfunction. Other events analyzed were the 2 individual components of the primary end point and cardiovascular mortality. Graft dysfunction was defined as any hospitalization due to clinical HF in the presence of a left ventricular ejection fraction (LVEF)<45%, determined by echocardiography or ventriculography, or of restrictive graft physiology, determined by echocardiography or an invasive hemodynamic study.18 Cardiovascular mortality was defined as that caused by HF, myocardial ischemia, or arrhythmia, including those deaths attributable to acute rejection, GVD, or any unexplained sudden death.
The causes of death were collected from autopsy reports and death certificates. Patients hospitalized due to graft dysfunction underwent a complete diagnostic work-up, including transthoracic echocardiography, coronary angiography, a hemodynamic study, and endomyocardial biopsy. A diagnosis of GVD was made in the presence of focal coronary stenosis>50% in a main epicardial vessel or diffuse concentric thickening of the entire vessel. Acute cellular rejection was considered the cause of graft dysfunction if it was classified as histological grade 2R or higher.19 In the absence of other causes, humoral rejection was considered to be the cause of graft dysfunction in patients who showed positive immunofluorescence for C4d with a pericapillary pattern.
Statistical AnalysisCategorical variables are presented as proportions, whereas continuous variables are presented as means (standard deviation). A Kolmogorov-Smirnov test was used to study the normality of the HRT values. Associations between baseline clinical characteristics and HRT were analyzed using Pearson's correlation coefficient for continuous variables, a Student t test for dichotomous qualitative variables, and analysis of variance with Bonferroni correction for qualitative variables with 3 or more categories.
Cox multivariate regression was used to determine the prognostic significance of the resting HRT at the end of the first post-HTx year. Based on clinical experience and the literature, the following candidate variables were selected for this analysis: donor age, recipient age, donor sex, recipient sex, diabetes mellitus, baseline heart disease and cytomegalovirus serological status, serum creatinine, and immunosuppression type. For each of the studied end points, a multivariate-adjusted model was constructed to include all variables whose entry or removal significantly changed the hazard ratio (HR) of the variable whose effect was the object of adjustment (HRT at the end of the first post-HTx year). Entry of the donor age was forced in all final models due to the correlation observed between this variable and heart rate. The variables retained in the final adjusted models were donor age, donor sex, and diabetes mellitus (death or graft dysfunction and all-cause mortality); donor age, donor sex, and recipient sex (cardiovascular mortality); and donor age (graft dysfunction).
For the analysis of follow-up events, patients were classified into 3 subgroups based on whether their HRT at the end of the first post-HTx year was found in the lower quartile, 2 middle quartiles, or upper quartile of the study population. Using the multivariate models described above, the adjusted HRs (aHRs) and cumulative incidence curves of the study events were calculated in these subgroups, considering as a reference category the patients with HRT in the 2 middle quartiles.
The HRT trend in the first 10 years after the HTx was studied using a repeated measures analysis of variance with Greenhouse-Geisser correction. In addition, the temporal trend in this parameter was estimated in each patient. The trend was considered increasing (a net increase in heart rate) in patients with a difference of>0 between the mean of all annual HRT measurements and the baseline determination. Otherwise, the trend was considered decreasing (a net reduction in heart rate). The previously constructed multivariate models were used to calculate the aHRs and cumulative incidence curves of study events for the subgroups of patients with increasing and decreasing HRT trends during follow-up. P<.05 was considered significant for all comparisons. Statistical analysis was performed with SPSS 20.
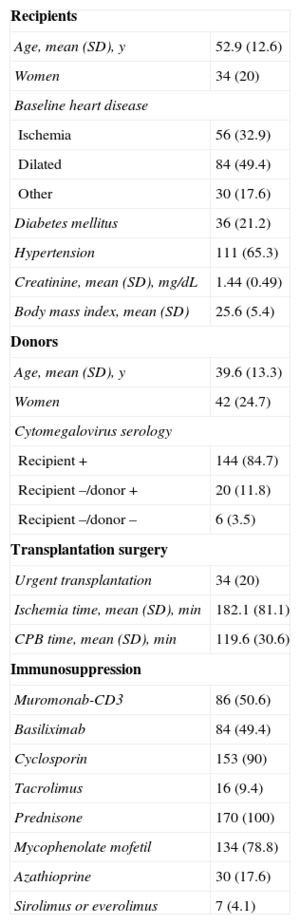
RESULTSPatientsBetween 1995 and 2005, 393 patients received an orthotopic HTx in our center. Of these, 322 survived at least 1 year after the intervention. A total of 152 patients were excluded from this study for the following reasons: treatment with negative chronotropes (n=132), simultaneous heart and renal transplantation (n=4), repeat HTx (n=2), severe anemia (n=2), and absence of analyzable electrocardiograms (n=12). The study population consisted of the 170 remaining patients. Their baseline clinical characteristics are shown in Table 1.
Clinical Characteristics of the Patients Included in the Study
| Recipients | |
| Age, mean (SD), y | 52.9 (12.6) |
| Women | 34 (20) |
| Baseline heart disease | |
| Ischemia | 56 (32.9) |
| Dilated | 84 (49.4) |
| Other | 30 (17.6) |
| Diabetes mellitus | 36 (21.2) |
| Hypertension | 111 (65.3) |
| Creatinine, mean (SD), mg/dL | 1.44 (0.49) |
| Body mass index, mean (SD) | 25.6 (5.4) |
| Donors | |
| Age, mean (SD), y | 39.6 (13.3) |
| Women | 42 (24.7) |
| Cytomegalovirus serology | |
| Recipient + | 144 (84.7) |
| Recipient –/donor + | 20 (11.8) |
| Recipient –/donor – | 6 (3.5) |
| Transplantation surgery | |
| Urgent transplantation | 34 (20) |
| Ischemia time, mean (SD), min | 182.1 (81.1) |
| CPB time, mean (SD), min | 119.6 (30.6) |
| Immunosuppression | |
| Muromonab-CD3 | 86 (50.6) |
| Basiliximab | 84 (49.4) |
| Cyclosporin | 153 (90) |
| Tacrolimus | 16 (9.4) |
| Prednisone | 170 (100) |
| Mycophenolate mofetil | 134 (78.8) |
| Azathioprine | 30 (17.6) |
| Sirolimus or everolimus | 7 (4.1) |
CPB, cardiopulmonary bypass; SD, standard deviation.
Unless otherwise indicated, values are expressed as No. (%).
At the end of the first post-HTx year, the resting HRT of the study population had a normal distribution (Kolmogorov-Smirnov test, P=.522). The mean HRT was 96.1 (SD, 1.4) bpm, and the first, second, third, and fourth quartiles were 55-89, 90-96, 97-104, and 105-132 bpm, respectively. The only baseline clinical variable showing a statistically significant correlation with HRT at the end of the first post-HTx year was donor age (r=–0.253; P=.001). The correlations between the different baseline clinical variables studied and HRT are shown in Tables 1 and 2 of the Supplementary Material.
Adverse EventsDuring a mean follow-up of 8.9 (SD, 3.1) years, 20 patients (11.8%) had graft dysfunction and 47 (27.6%) died. The composite primary study end point occurred in 55 patients (32.3%). Graft dysfunction was attributed to GVD in 10 patients, to humoral rejection in 4, and cellular rejection in 3. No specific cause of the graft dysfunction could be identified in the 3 remaining patients.
In addition, 23 deaths (48.9%) were attributed to cardiac causes: refractory HF in 7 patients (secondary to GVD in 5 patients and to cellular rejection in 2 patients) and sudden death in 16 patients (5 of these had a previous diagnosis of GVD). Noncardiovascular mortality was due to neoplasms (n=13), infection (n=8), liver disease (n=1), drug abuse (n=1), and hemorrhage (n=1).
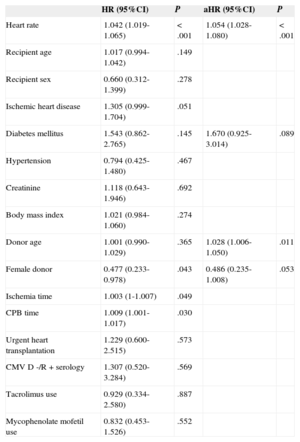
Prognostic Value of Heart RateThe univariate Cox analysis showed a statistically significant association between a higher resting HRT at the end of the first post-HTx year and the incidence of the primary composite end point (crude HR=1.042; 95% confidence interval [95%CI], 1.019-1.065; P<.001). This association continued to be significant (aHR=1.054; 95%CI, 1.028-1.080; P<.001) following the multivariate adjustment (Table 2). In addition, a significant association was seen between HRT and the risk of all-cause mortality (aHR=1.058; 95%CI, 1.030-1.087; P=.001), as well as the risk of cardiovascular mortality (aHR=1.069; 95%CI, 1.026-1.113; P<.001). There was no statistically significant association between HRT and the risk of graft dysfunction (aHR=1.028; 95%CI, 0.989-1.069; P=.161).
Clinical Variables Associated with the Primary Composite End Point of Death or Graft Dysfunction: Multivariate Cox Proportional Hazards Analysis
| HR (95%CI) | P | aHR (95%CI) | P | |
|---|---|---|---|---|
| Heart rate | 1.042 (1.019-1.065) | <.001 | 1.054 (1.028-1.080) | <.001 |
| Recipient age | 1.017 (0.994-1.042) | .149 | ||
| Recipient sex | 0.660 (0.312-1.399) | .278 | ||
| Ischemic heart disease | 1.305 (0.999-1.704) | .051 | ||
| Diabetes mellitus | 1.543 (0.862-2.765) | .145 | 1.670 (0.925-3.014) | .089 |
| Hypertension | 0.794 (0.425-1.480) | .467 | ||
| Creatinine | 1.118 (0.643-1.946) | .692 | ||
| Body mass index | 1.021 (0.984-1.060) | .274 | ||
| Donor age | 1.001 (0.990-1.029) | .365 | 1.028 (1.006-1.050) | .011 |
| Female donor | 0.477 (0.233-0.978) | .043 | 0.486 (0.235-1.008) | .053 |
| Ischemia time | 1.003 (1-1.007) | .049 | ||
| CPB time | 1.009 (1.001-1.017) | .030 | ||
| Urgent heart transplantation | 1.229 (0.600-2.515) | .573 | ||
| CMV D -/R + serology | 1.307 (0.520-3.284) | .569 | ||
| Tacrolimus use | 0.929 (0.334-2.580) | .887 | ||
| Mycophenolate mofetil use | 0.832 (0.453-1.526) | .552 |
95%CI, 95% confidence interval; aHR, adjusted hazard ratio; CMV, cytomegalovirus; CPB, cardiopulmonary bypass; D, donor; HR, crude hazard ratio; R, recipient.
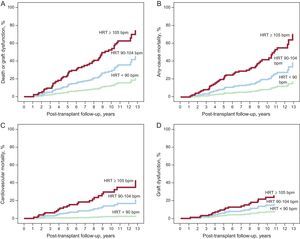
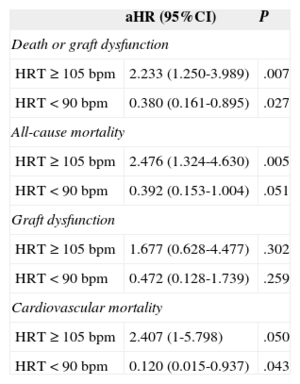
The cumulative incidence curves of each of the study end points in the subgroups of patients with HRT in the lower quartile (< 90 bpm), in the 2 middle quartiles (90-104 bpm), and in the upper quartile (≥ 105 bpm) are shown in Figure 1. Compared with the middle reference category, the aHRs for the main composite end point were 2.233 (95%CI, 1.250-3.989; P=.007) and 0.380 (95CI%, 0.161-0.895; P=.027) in patients with heart rates ≥ 105 and<90 bpm, respectively. The aHRs for the other study end points are shown in Table 3.
Multivariate Cox Proportional Hazards Analysis
| aHR (95%CI) | P | |
|---|---|---|
| Death or graft dysfunction | ||
| HRT ≥ 105 bpm | 2.233 (1.250-3.989) | .007 |
| HRT<90 bpm | 0.380 (0.161-0.895) | .027 |
| All-cause mortality | ||
| HRT ≥ 105 bpm | 2.476 (1.324-4.630) | .005 |
| HRT<90 bpm | 0.392 (0.153-1.004) | .051 |
| Graft dysfunction | ||
| HRT ≥ 105 bpm | 1.677 (0.628-4.477) | .302 |
| HRT<90 bpm | 0.472 (0.128-1.739) | .259 |
| Cardiovascular mortality | ||
| HRT ≥ 105 bpm | 2.407 (1-5.798) | .050 |
| HRT<90 bpm | 0.120 (0.015-0.937) | .043 |
95%CI, 95% confidence interval; aHR, adjusted hazard ratio; bpm, beats per minute; HRT, heart rate.
aHRs of the study end points in 3 resting heart rate categories at the end of the first post-transplant year. Risk estimation was performed with respect to the reference category of patients with a heart rate within the 2 middle quartiles (90-104 bpm).
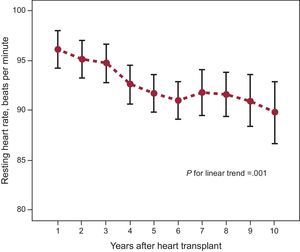
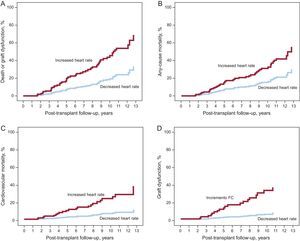
During the first 10 years of follow-up after the HTx, the mean HRT of the study population showed a linear decreasing trend (P=.001) (Figure 2). Patients showing a net increase in HRT during follow-up had a significantly higher incidence of the primary composite end point (aHR=2.857; 95%CI, 1.514-5.391; P=.001) than patients with a net decrease in heart rate. The risks of all-cause mortality (aHR=2.104; 95%CI, 1.069-4.142; P=.031), graft dysfunction (aHR=6.839; 95%CI, 2.371-19.730; P<.001), and cardiovascular mortality (aHR=4.051; 95%CI, 1.536-10.684; P=.005) were also significantly higher in patients with a net increase in heart rate. The cumulative incidence curves of end points based on the change in the HRT over time are shown in Figure 3.
The present study supports the prognostic value of HRT in HTx patients. In our series, the presence of a higher resting HRT at the end of the first post-HTx year was independently associated with an increased cumulative incidence of the composite end point of all-cause mortality and graft dysfunction. This result was due to increased overall mortality; however, the tendency toward a higher risk of graft dysfunction failed to reach statistical significance, probably due to the small number of events and the low statistical power for the individual analysis of these events. Notably, the prognostic significance of HRT was not limited to a single measurement, because the temporal tendency of the parameter was shown to be a prognostic marker independently of the baseline values.
Heart transplant operations necessitate transection of the autonomic fibers innervating the native heart. Due to the lack of parasympathetic stimulation, the HRT of transplanted hearts are largely determined by their response to circulating catecholamines.11 Compared with healthy controls, HTx recipients show a persistent elevation of resting HRT with limited circadian variability and a delayed response to exercise.11 A “normal” HRT remains to be clearly defined, with mean values in different series varying between 85 and 100 bpm.12–17 This apparent variability is due to differences in surgical techniques, donor age, and drug treatment, as well as the time since the HTx, because some patients experience a gradual reinnervation phenomenon of the graft that progressively reduces the heart rate.20
At the end of the first post-HTx year, the resting HRT values in our population were normally distributed, with mean and median coinciding at 96 bpm and a middle interquartile range of 90 to 104 bpm. In relation with this reference category, patients with a HRT ≥ 105 bpm showed an increase of more than twice the incidence of the primary end point of death or graft dysfunction, whereas this incidence was reduced to a similar extent in those patients with a HRT<90 bpm. Similarly, Anand et al12 and Castel et al14 observed significantly lower survival in HTx patients with a resting HRT>90 bpm than in those with a HRT<90 bpm, whereas Melero-Ferrer et al13 reached a similar conclusion using a 100 bpm threshold. Measurement of HRT was performed at 1 post-HTx year in 2 of these studies13,14 and at 3 months in the other.12
The negative prognostic impact of the elevated HRT values seen in our study is attributable to an increased risk of cardiovascular mortality, mainly refractory HF and sudden death. The most frequent underlying heart disease in these patients was GVD, which was the cause of death in close to half of the patients who died of cardiac causes. However, the absence of systematic coronary angiographies in patients who received transplants during the first years of the study hinders a more accurate estimation of the impact of GVD on cause of death in our population. In fact, the rate of GVD was likely higher than that detected, given the high proportion of unexplained sudden deaths. In the study by Castel et al,14 the lower survival of HTx recipients with higher HRT values was attributed to an increased mortality from GVD. However, Anand et al12 failed to see any association between resting HRT and the distribution of the causes of death. No specific information on causes of mortality was provided in the study of Melero-Ferrer et al.13
The reasons for the association between HRT and risk of death remain to be determined in HTx patients. In some cases, the high HRT can be attributed to an adaptive reaction21 to an underlying adverse clinical condition such as hypovolemia, anemia, graft dysfunction, bronchopulmonary disease, infection, or neoplasia, so that it should be interpreted as a risk marker rather than a strict risk factor. Nevertheless, permanent tachycardia could by itself play a causal role in the development of contractile dysfunction of the graft, mediated by myocardial energy depletion.21 In other cases, the HRT elevation can reflect a high concentration of circulating catecholamines, which increase the risk of hypertension, myocardial ischemia, adverse ventricular remodeling, and arrhythmogenesis.22 In addition, it must be remembered that persistent HRT elevation in patients with a native heart is implicated in the genesis and progression of coronary artery disease.23 High HRT stimulates the endothelial expression of adhesion molecules and cytokines such as interleukin-6 and tumor necrosis factor-alpha,24 which can hypothetically promote an immunological response against the coronary vessels of the graft. Nevertheless, studies have yet to show a clear association between a persistently high HRT and increased risk of GVD in HTx recipients.15–17
The natural history of HTx patients includes a gradual reinnervation of the graft with a progressively reduced resting HRT and improved response of HRT to exercise.20,21 The mean HRT in our population gradually fell in the first 10 years after HTx. Notably, the incidence of the primary composite end point was significantly higher in patients with a net increase in HRT in this period than in those who experienced the expected net decrease. Anand et al12 described a similar association between a decreasing trend in the resting HRT and a lower long-term rate of mortality. The progressive HRT decrease after HTx likely reflects the recovery of effective parasympathetic innervation of the graft. This phenomenon counteracts the deleterious effects of chronic adrenergic stimulation and is thus considered important for long-term maintenance of the contractile function of the transplanted heart.21,25 Nonetheless, parasympathetic reinnervation of the graft is less frequent than sympathetic reinnervation.26,27
Prognostic benefit has been seen in HF and coronary disease patients chronically treated with heart rate-reducing drugs, such as beta-blockers and ivabradine.8–10 However, their efficacy in HTx recipients remains unknown. In general, treatment of these patients with beta-blockers is discouraged, because these agents can worsen the contractile function of the graft and exercise capacity.28,29 A preliminary study indicated that diltiazem use during the first post-HTx year could slow the progression of GVD,30 but this benefit was not subsequently confirmed. Recently, a small study showed that ivabradine is a safe drug, well tolerated and effective for reducing HRT in HTx patients in sinus rhythm,31 but there are still no conclusive data on its potential clinical benefit in this population.
The present study has some limitations. Due to its observational and retrospective design, the study could be affected by selection and information biases inherent to this type of research. In addition, despite the use of rigorous multivariate adjustment, we are unable to rule out a possible effect of another untested confounding factor on the observed association between HRT and adverse events. The absence of information on the onset of de novo tachyarrhythmias during follow-up is another limitation, even if it is unlikely to significantly impact the observed results because of its low incidence in patients undergoing surgery using the bicaval technique.32 Finally, the limited context of this single-center study indicates that its external relevance cannot be guaranteed and that the findings should be confirmed in larger multicenter populations.
Compared with previous studies examining the relationship between HRT and prognosis following HTx (all single center and with small sample sizes),12–14 the main strengths of this work are its long follow-up, the rigorous selection criteria (eg, use of the same surgical technique in all patients, exclusion of patients with drugs or comorbidity that could affect heart rate), the analysis of other end points besides total mortality (eg, graft dysfunction, cardiovascular mortality), and the description of the temporal trend in HRT and its prognostic implication.
CONCLUSIONSThe present study confirms the existence of a strong association between higher resting HRT values and worse long-term prognosis in HTx patients. This association is largely due to an increased risk of cardiovascular mortality, mainly GVD, through sudden death and refractory HF. According to our results, the prognosis is particularly adverse for patients with a markedly higher resting HRT (> 105 bpm) at the end of the first post-HTx year and those whose HRT tended to increase over time. This study supports the introduction of resting HRT as a simple and easily measured marker of risk in the clinical management of HTx patients and indicates the need for new studies evaluating the potential clinical benefit of heart rate-reducing drugs in this population. The confirmation of the favorable long-term prognosis shown by HTx patients with a resting HRT<90 bpm, in line with that observed in previous studies, supports this cutoff value as a hypothetical therapeutic target in future intervention studies.
CONFLICTS OF INTERESTNone.