There is little evidence on the prognostic influence of intravenous nitrates in patients with acute heart failure. Our purpose was to determine the influence of this treatment on early mortality and new visits.
MethodsProspective, multicenter cohort study of patients with acute heart failure in an emergency room during 2 periods (May 2009 and November-December 2011). Patients with systolic blood pressure > 110mmHg were included, grouped according to whether they received intravenous nitroglycerin or not. Endpoints were mortality at 3, 7, 14, and 30 days and new visits at 30 days. The propensity score was estimated by logistic regression to determine the prognostic influence of the treatment.
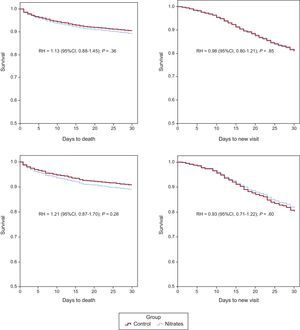
ResultsWe included 3178 of 4897 individuals. A total of 308 (9.7%) had died within 30 days and 465 (17%) attended new visits. The mean (standard deviation) age was 79.5 (10.0) years, and 796 (25%) patients received intravenous nitrates. After matching, there were 685 individuals in each group. The hazard ratio for 30-day mortality with nitrates was 1.21 (95% confidence interval, 0.87-1.70) and was 0.93 for new visits (95% confidence interval, 0.71-1.22). The results were similar for mortality at 3, 7, and 14 days (hazard ratio = 1.05 [95% confidence interval, 0.56-1.96], hazard ratio = 1.20 [95% confidence interval, 0.74-1.94], and hazard ratio = 1.23 [95% confidence interval, 0.82-1.84], respectively). In the presence of hypertensive pulmonary edema, the nitrates group showed a hazard ratio of 0.88 (95% confidence interval, 0.47-1.63) for 30-day mortality.
ConclusionsIntravenous nitrates do not influence early mortality or new visits in patients with acute heart failure.
Keywords
Acute heart failure (AHF) is the leading cause of hospitalization and consultation in hospital emergency rooms (ERs) in industrialized nations,1–3 and its prevalence increases with age.1,4 Treatment is mainly undertaken to restore oxygenation and improve infusion, and intravenous (IV) nitrates are one of the essential drugs used for this purpose. These drugs are administered in 6% to 70% of patients5 because the scientific evidence for vasodilators in AHF is very limited.6,7
The European Society of Cardiology guidelines6 recommend the use of IV nitroglycerin in patients with pulmonary congestion/edema and systolic blood pressure (SBP) > 110mmHg without severe aortic or mitral stenosis, in order to decrease pulmonary capillary pressure and systemic vascular resistance (grade of recommendation IIa and level of evidence B). These drugs reduce preloading and postloading and increase systolic volume, although there are no data that clearly show that they alleviate dyspnea or improve other clinical results, such as mortality.8 The most commonly used vasodilator is nitroglycerin, which is usually administered through the IV route but can be used sublingually in earlier phases.9,10 A Cochrane Library review on the use of nitrates in AHF syndromes11 found only 4 studies, which included a total of 634 patients: 2 were studies of patients who developed AHF after an acute myocardial infarction, another excluded patients with acute myocardial infarction, and the fourth included patients with and without acute coronary syndrome. The review concluded that the studies were of very poor quality and did not allow robust evidence to be obtained. Mortality has been measured in a study that compared IV nitroglycerin vs nesiritide, and no differences were found in 30-day mortality.12 In this regard, a 3CPO trial substudy showed that the use of IV nitrates did not improve mortality in patients with acute pulmonary edema (APE).13 In summary, these drugs have a clinical effect on the decrease of dyspnea and the improvement in hemodynamic parameters, but it is not known how they affect early mortality because there are no clinical trial data on this topic. Therefore, we aimed to identify the profile of patients with AHF seen in hospital ERs who receive IV nitrates and to determine the effect of this treatment on early mortality and new visits for AHF. The null hypothesis was that nitrate use does not influence early mortality in patients with AHF.
METHODSStudy TypeThe EAHFE registry9 is a multicenter, prospective, noninterventional cohort study with consecutive inclusion of patients with AHF attended at 29 Spanish hospital ERs. The inclusion criterion was compliance with the Framingham diagnostic criteria. Patients subsequently received follow-up, and new ER visits for additional episodes of AHF and all-cause death were recorded. During the design phase of the inclusion period, the authors took into account the analysis of patients who received IV nitrate treatment in the acute phase, in order to understand their clinical profile and determine whether nitrate use is associated with a better short-term prognosis.
The study was designated NITRO-EAHFE and was conducted in accordance with the Declaration of Helsinki of 2010 on Ethical Principles for Medical Research Involving Human Subjects, and all patients gave consent to participate. The complete protocol was approved by the clinical research ethics committees of the participating hospitals.
The NITRO-EAHFE study included patients who had SBP > 110mmHg when they presented to the ER, had completed follow-up, and had valid values for the variables considered relevant. The patients were divided into 2 groups according to whether they had been treated (nitrates group) or not (control group) with IV nitroglycerin during the episode. Treatment was decided based on purely clinical criteria determined by the physician responsible for the patient's care in the ER and on the fact that all sites follow the European Society of Cardiology recommendations.1,6 Intravenous nitroglycerin was prescribed according to an established protocol, as part of the patient's initial care in the hospital ER; specifically, a 50-mg ampoule of Solinitrina Forte® (nitroglycerin) was diluted in 250mL of 5% glucose saline and a continuous infusion was started at a dose of 5 to 200mg/min, depending on SBP. The infusion was maintained for 6 hours to 48hours, at the investigator's discretion and depending on the clinical response and SBP figures.
Demographic, Clinical, and Additional Examination DataAll demographic, clinical, and additional examination data collected in the ER were recorded. The demographic data included age and sex, whereas the comorbidity data included a history of hypertension, diabetes mellitus, dyslipidemia, ischemic heart disease, valve disease, atrial fibrillation, chronic kidney disease, cerebrovascular disease, chronic obstructive pulmonary disease, peripheral artery disease, or prior AHF episodes. Data on the patients’ baseline functional situation included functional status as measured by the Barthel index14 and functional class for dyspnea according to the New York Heart Association classification. The following were collected for acute episodes: SBP, heart and respiratory rates, baseline arterial oxygen saturation by pulse oximetry, electrocardiogram rhythm, and whether the episode presented as hypertensive APE according to the European Society of Cardiology definition.1 The analytical data included hemoglobin (anemia defined according to the World Health Organization recommendations as hemoglobin < 12g/L in women and < 13g/L in men), glucose, creatinine, urea, and sodium. The estimated glomerular filtration rate was calculated by the abbreviated formula of the Modification of Diet in Renal Disease study.15 Data were collected on previous treatment and patient management in hospital ERs according to treatment administered and destination after ER care (admission or discharge).
Follow-up and MortalityThe follow-up endpoints were mortality at 3, 7, 14, and 30 days and new visits at 30 days from study inclusion. Follow-up was done by a telephone call and/or consultation of the patient's hospital or primary-care medical history.
Statistical AnalysisFor the description, absolute and relative frequencies were used for qualitative variables, and the mean with standard deviation or the median and interquartile range were used for quantitative variables. For raw comparisons between patients treated with or without nitroglycerin, the chi-square test was used for the initial comparisons and the Student t test was used for the second comparisons; the nonparametric Mann-Whitney U test was used if normal criteria were not met, which was then contrasted by the Kolmogorov-Smirnov test.
To determine the influence of IV nitrate therapy on mortality, a propensity score analysis was performed using a logistic regression model, which included variables with P < .20 in the bivariate comparison between patients who received nitrates and patients who had not. Other variables without this P value were also included if they were considered a priori to be relevant by the research team based on organizational, epidemiologic, or clinical criteria. A logistic regression analysis was performed, and the probability was calculated. Subsequently, individuals were matched with a probability difference < 0.05, and a control patient was selected for each patient in the nitrates group (further information on the procedure is included in Appendix 2 of the supplementary material). The Cox proportional-hazards model was used for the analysis and survival charts, and the hazard ratios (HR) were calculated with their respective 95% confidence intervals (95%CI) for 30-day mortality and new visits, for both the original groups and the matched group. For the case of mortality, the survival curves truncated at 3, 7, and 14 days were also analyzed individually. In addition, these analyses were repeated once patients with a clinical diagnosis of hypertensive APE were separated from patients without this diagnosis. Differences were considered to be statistically significant when P < .05. SPSS 18.0 and R3.1 were used for the statistical analysis.
RESULTSOf a total of 4897 individuals in the EAHFE 2 and 3 registries, 607 had SBP ≤ 110mmHg, 671 were lost to follow-up, and 441 were missing at least 1 essential variable (Figure 1). These patients were not included in the definitive analysis but showed no differences compared with the remaining patients in the medical data for the episode. A final total of 3178 patients were included in the NITRO-EAHFE study: 796 (25%) received IV nitrate therapy during their care in hospital ERs and were included in the nitrates group and the remaining 2382 (75%) were assigned to the control group. The 30-day global mortality was 308 (9.7%) patients, 84 (10.6%) in the nitrates group and 224 (9.4%) in the controL group, and new visits was 465 (17%) for the whole group and 117 (17.3%) and 348 (16.9%) in the nitrates and control groups, respectively. In the matched patients, 30-day mortality was 75 (10.9%) in the nitrates group and 62 (9.1%) in the control group and 30-day new visits was 97 (16.9%) and 107 (17.8%), respectively.
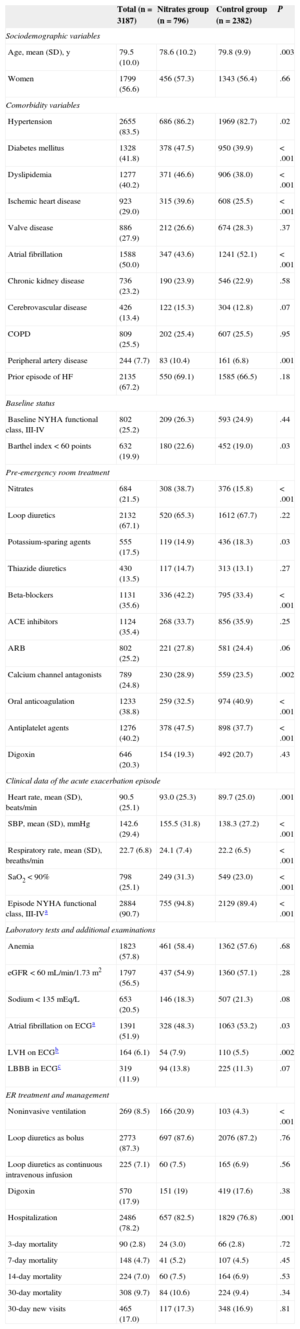
Table 1 lists the baseline characteristics for the entire sample and a comparison of both groups. A total of 56.6% of the entire sample were women, and the mean (standard deviation) age was 79.5 (10.0) years. The most prevalent risk factor was hypertension (83.5% of patients), and the most common associated diseases included a history of heart failure (67.2%) and atrial fibrillation (50%). A total of 25.2% of patients had a baseline New York Heart Association functional class of III to IV for dyspnea and 19.9% had a severe functional dependence with a Barthel index < 60 points. Regarding long-term therapy before the hospital ER visit, 67.1% were receiving loop diuretics, 35.4% were receiving angiotensin-converting enzyme inhibitors, and 35.6% were receiving beta-blockers. Based on episode data, 90.7% had a New York Heart Association functional class of III to IV, 56.5% had an estimated glomerular filtration rate < 60mL/min/1.73 m2, 57.8% had anemia, and 51.9% had atrial fibrillation. Among the treatments received in the acute phase, the most common was loop diuretic as bolus (87.3% of patients); 8.5% required noninvasive ventilation, and 7% required IV morphine.
Baseline Characteristics of the Global Patient Sample and the 2 Groups (Nitrates Group and Control Group)
| Total (n = 3187) | Nitrates group (n = 796) | Control group (n = 2382) | P | |
|---|---|---|---|---|
| Sociodemographic variables | ||||
| Age, mean (SD), y | 79.5 (10.0) | 78.6 (10.2) | 79.8 (9.9) | .003 |
| Women | 1799 (56.6) | 456 (57.3) | 1343 (56.4) | .66 |
| Comorbidity variables | ||||
| Hypertension | 2655 (83.5) | 686 (86.2) | 1969 (82.7) | .02 |
| Diabetes mellitus | 1328 (41.8) | 378 (47.5) | 950 (39.9) | < .001 |
| Dyslipidemia | 1277 (40.2) | 371 (46.6) | 906 (38.0) | < .001 |
| Ischemic heart disease | 923 (29.0) | 315 (39.6) | 608 (25.5) | < .001 |
| Valve disease | 886 (27.9) | 212 (26.6) | 674 (28.3) | .37 |
| Atrial fibrillation | 1588 (50.0) | 347 (43.6) | 1241 (52.1) | < .001 |
| Chronic kidney disease | 736 (23.2) | 190 (23.9) | 546 (22.9) | .58 |
| Cerebrovascular disease | 426 (13.4) | 122 (15.3) | 304 (12.8) | .07 |
| COPD | 809 (25.5) | 202 (25.4) | 607 (25.5) | .95 |
| Peripheral artery disease | 244 (7.7) | 83 (10.4) | 161 (6.8) | .001 |
| Prior episode of HF | 2135 (67.2) | 550 (69.1) | 1585 (66.5) | .18 |
| Baseline status | ||||
| Baseline NYHA functional class, III-IV | 802 (25.2) | 209 (26.3) | 593 (24.9) | .44 |
| Barthel index < 60 points | 632 (19.9) | 180 (22.6) | 452 (19.0) | .03 |
| Pre-emergency room treatment | ||||
| Nitrates | 684 (21.5) | 308 (38.7) | 376 (15.8) | < .001 |
| Loop diuretics | 2132 (67.1) | 520 (65.3) | 1612 (67.7) | .22 |
| Potassium-sparing agents | 555 (17.5) | 119 (14.9) | 436 (18.3) | .03 |
| Thiazide diuretics | 430 (13.5) | 117 (14.7) | 313 (13.1) | .27 |
| Beta-blockers | 1131 (35.6) | 336 (42.2) | 795 (33.4) | < .001 |
| ACE inhibitors | 1124 (35.4) | 268 (33.7) | 856 (35.9) | .25 |
| ARB | 802 (25.2) | 221 (27.8) | 581 (24.4) | .06 |
| Calcium channel antagonists | 789 (24.8) | 230 (28.9) | 559 (23.5) | .002 |
| Oral anticoagulation | 1233 (38.8) | 259 (32.5) | 974 (40.9) | < .001 |
| Antiplatelet agents | 1276 (40.2) | 378 (47.5) | 898 (37.7) | < .001 |
| Digoxin | 646 (20.3) | 154 (19.3) | 492 (20.7) | .43 |
| Clinical data of the acute exacerbation episode | ||||
| Heart rate, mean (SD), beats/min | 90.5 (25.1) | 93.0 (25.3) | 89.7 (25.0) | .001 |
| SBP, mean (SD), mmHg | 142.6 (29.4) | 155.5 (31.8) | 138.3 (27.2) | < .001 |
| Respiratory rate, mean (SD), breaths/min | 22.7 (6.8) | 24.1 (7.4) | 22.2 (6.5) | < .001 |
| SaO2 < 90% | 798 (25.1) | 249 (31.3) | 549 (23.0) | < .001 |
| Episode NYHA functional class, III-IVa | 2884 (90.7) | 755 (94.8) | 2129 (89.4) | < .001 |
| Laboratory tests and additional examinations | ||||
| Anemia | 1823 (57.8) | 461 (58.4) | 1362 (57.6) | .68 |
| eGFR < 60 mL/min/1.73 m2 | 1797 (56.5) | 437 (54.9) | 1360 (57.1) | .28 |
| Sodium < 135 mEq/L | 653 (20.5) | 146 (18.3) | 507 (21.3) | .08 |
| Atrial fibrillation on ECGa | 1391 (51.9) | 328 (48.3) | 1063 (53.2) | .03 |
| LVH on ECGb | 164 (6.1) | 54 (7.9) | 110 (5.5) | .002 |
| LBBB in ECGc | 319 (11.9) | 94 (13.8) | 225 (11.3) | .07 |
| ER treatment and management | ||||
| Noninvasive ventilation | 269 (8.5) | 166 (20.9) | 103 (4.3) | < .001 |
| Loop diuretics as bolus | 2773 (87.3) | 697 (87.6) | 2076 (87.2) | .76 |
| Loop diuretics as continuous intravenous infusion | 225 (7.1) | 60 (7.5) | 165 (6.9) | .56 |
| Digoxin | 570 (17.9) | 151 (19) | 419 (17.6) | .38 |
| Hospitalization | 2486 (78.2) | 657 (82.5) | 1829 (76.8) | .001 |
| 3-day mortality | 90 (2.8) | 24 (3.0) | 66 (2.8) | .72 |
| 7-day mortality | 148 (4.7) | 41 (5.2) | 107 (4.5) | .45 |
| 14-day mortality | 224 (7.0) | 60 (7.5) | 164 (6.9) | .53 |
| 30-day mortality | 308 (9.7) | 84 (10.6) | 224 (9.4) | .34 |
| 30-day new visits | 465 (17.0) | 117 (17.3) | 348 (16.9) | .81 |
ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blockers; COPD, chronic obstructive pulmonary disease; ECG, electrocardiogram; eGFR, estimated glomerular filtration rate; ER, emergency room; HF, heart failure; LBBB, left bundle-branch block; LVH, left ventricular hypertrophy; NYHA, New York Heart Association; SaO2, arterial oxygen saturation; SBP, systolic blood pressure; SD, standard deviation.
Data are expressed as No. (%) or mean (standard deviation).
A comparison of the groups showed that the patients in the NITRATES group were younger (78.6 vs 79.8 years of age; P = .003), with a higher prevalence of diabetes mellitus (47.2% vs 39.9%; P < .001), dyslipidemia (46.6% vs 38%; P < .001) and previous ischemic heart disease (39.6% vs 25.5%; P < .001), and a lower prevalence of previous atrial fibrillation (43.6% vs 52.1%; P < .001) and baseline functional dependence (22.6% with Barthel < 60 points vs 19%; P < .03). In the case of home treatment, the nitrates group was largely with oral or transdermal nitrates (38.7% vs 15.8%; P < .001) and beta-blockers (42.2% vs 33.4%; P < .001), and had a lower proportion with oral anticoagulation (32.5% vs 40.9%; P < .001). Based on the clinical data of the acute episode, the patients in the nitrates group had a faster heart rate (93 bpm vs 89.7 bpm; P = .001), SBP (155.5mmHg vs 138.3mmHg; P < .0001) and more respiratory failure, with a higher percentage of them with arterial oxygen saturation < 90% and faster respiratory rate (P < .001 in both cases). In the case of ER treatment, the nitrates group were more likely to receive noninvasive ventilation (20.9% vs 4.3%; P < .001) and were hospitalized more often (82.5% vs 76.8%; P < .001).
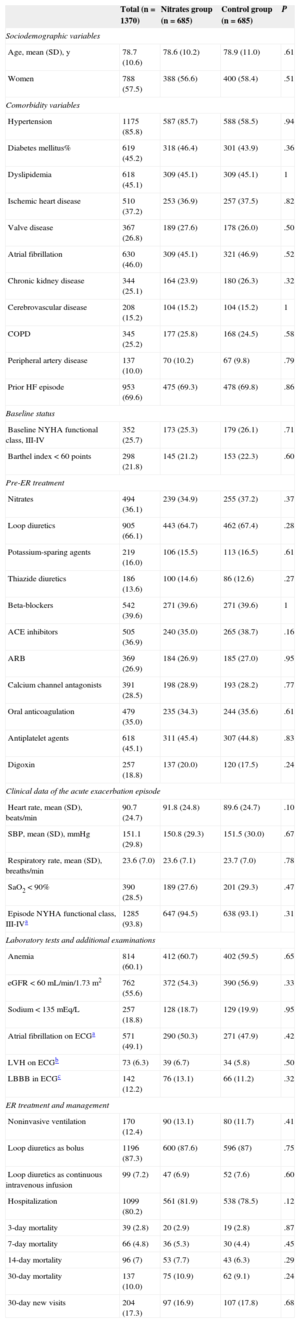
After propensity score matching, 685 individuals remained in each group. The value of the c-statistic of the propensity score logistic model was 0.77 (95%CI, 0.75-0.79). The analysis between the matched groups showed no differences in any of the variables between the nitrates and control groups created (Table 2). The raw HR values for 30-day mortality and new visits for the nitrates group were 1.13 (95%CI, 0.88-1.45) and 0.98 (95%CI, 0.80-1.21), respectively. The HR values after matching were 1.21 (95%CI, 0.87-1.70) for 30-day mortality and 0.93 (95%CI, 0.71-1.22) for 30-day readmission (Figure 1). The results were similar when mortality at 3, 7, and 14 days was analyzed, with HR 1.05 (95%CI, 0.56-1.96), 1.20 (95%CI, 0.74-1.94), and 1.23 (95%CI, 0.82-1.84), respectively.
Baseline Characteristics and Postmatching Characteristics of the 2 Groups (Nitrates Group and Control Group) Following Propensity Score Matching
| Total (n = 1370) | Nitrates group (n = 685) | Control group (n = 685) | P | |
|---|---|---|---|---|
| Sociodemographic variables | ||||
| Age, mean (SD), y | 78.7 (10.6) | 78.6 (10.2) | 78.9 (11.0) | .61 |
| Women | 788 (57.5) | 388 (56.6) | 400 (58.4) | .51 |
| Comorbidity variables | ||||
| Hypertension | 1175 (85.8) | 587 (85.7) | 588 (58.5) | .94 |
| Diabetes mellitus% | 619 (45.2) | 318 (46.4) | 301 (43.9) | .36 |
| Dyslipidemia | 618 (45.1) | 309 (45.1) | 309 (45.1) | 1 |
| Ischemic heart disease | 510 (37.2) | 253 (36.9) | 257 (37.5) | .82 |
| Valve disease | 367 (26.8) | 189 (27.6) | 178 (26.0) | .50 |
| Atrial fibrillation | 630 (46.0) | 309 (45.1) | 321 (46.9) | .52 |
| Chronic kidney disease | 344 (25.1) | 164 (23.9) | 180 (26.3) | .32 |
| Cerebrovascular disease | 208 (15.2) | 104 (15.2) | 104 (15.2) | 1 |
| COPD | 345 (25.2) | 177 (25.8) | 168 (24.5) | .58 |
| Peripheral artery disease | 137 (10.0) | 70 (10.2) | 67 (9.8) | .79 |
| Prior HF episode | 953 (69.6) | 475 (69.3) | 478 (69.8) | .86 |
| Baseline status | ||||
| Baseline NYHA functional class, III-IV | 352 (25.7) | 173 (25.3) | 179 (26.1) | .71 |
| Barthel index < 60 points | 298 (21.8) | 145 (21.2) | 153 (22.3) | .60 |
| Pre-ER treatment | ||||
| Nitrates | 494 (36.1) | 239 (34.9) | 255 (37.2) | .37 |
| Loop diuretics | 905 (66.1) | 443 (64.7) | 462 (67.4) | .28 |
| Potassium-sparing agents | 219 (16.0) | 106 (15.5) | 113 (16.5) | .61 |
| Thiazide diuretics | 186 (13.6) | 100 (14.6) | 86 (12.6) | .27 |
| Beta-blockers | 542 (39.6) | 271 (39.6) | 271 (39.6) | 1 |
| ACE inhibitors | 505 (36.9) | 240 (35.0) | 265 (38.7) | .16 |
| ARB | 369 (26.9) | 184 (26.9) | 185 (27.0) | .95 |
| Calcium channel antagonists | 391 (28.5) | 198 (28.9) | 193 (28.2) | .77 |
| Oral anticoagulation | 479 (35.0) | 235 (34.3) | 244 (35.6) | .61 |
| Antiplatelet agents | 618 (45.1) | 311 (45.4) | 307 (44.8) | .83 |
| Digoxin | 257 (18.8) | 137 (20.0) | 120 (17.5) | .24 |
| Clinical data of the acute exacerbation episode | ||||
| Heart rate, mean (SD), beats/min | 90.7 (24.7) | 91.8 (24.8) | 89.6 (24.7) | .10 |
| SBP, mean (SD), mmHg | 151.1 (29.8) | 150.8 (29.3) | 151.5 (30.0) | .67 |
| Respiratory rate, mean (SD), breaths/min | 23.6 (7.0) | 23.6 (7.1) | 23.7 (7.0) | .78 |
| SaO2 < 90% | 390 (28.5) | 189 (27.6) | 201 (29.3) | .47 |
| Episode NYHA functional class, III-IVa | 1285 (93.8) | 647 (94.5) | 638 (93.1) | .31 |
| Laboratory tests and additional examinations | ||||
| Anemia | 814 (60.1) | 412 (60.7) | 402 (59.5) | .65 |
| eGFR < 60 mL/min/1.73 m2 | 762 (55.6) | 372 (54.3) | 390 (56.9) | .33 |
| Sodium < 135 mEq/L | 257 (18.8) | 128 (18.7) | 129 (19.9) | .95 |
| Atrial fibrillation on ECGa | 571 (49.1) | 290 (50.3) | 271 (47.9) | .42 |
| LVH on ECGb | 73 (6.3) | 39 (6.7) | 34 (5.8) | .50 |
| LBBB in ECGc | 142 (12.2) | 76 (13.1) | 66 (11.2) | .32 |
| ER treatment and management | ||||
| Noninvasive ventilation | 170 (12.4) | 90 (13.1) | 80 (11.7) | .41 |
| Loop diuretics as bolus | 1196 (87.3) | 600 (87.6) | 596 (87) | .75 |
| Loop diuretics as continuous intravenous infusion | 99 (7.2) | 47 (6.9) | 52 (7.6) | .60 |
| Hospitalization | 1099 (80.2) | 561 (81.9) | 538 (78.5) | .12 |
| 3-day mortality | 39 (2.8) | 20 (2.9) | 19 (2.8) | .87 |
| 7-day mortality | 66 (4.8) | 36 (5.3) | 30 (4.4) | .45 |
| 14-day mortality | 96 (7) | 53 (7.7) | 43 (6.3) | .29 |
| 30-day mortality | 137 (10.0) | 75 (10.9) | 62 (9.1) | .24 |
| 30-day new visits | 204 (17.3) | 97 (16.9) | 107 (17.8) | .68 |
ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blockers; COPD, chronic obstructive pulmonary disease; ECG, electrocardiogram; eGFR, estimated glomerular filtration rate; ER, emergency room; HF, heart failure; LBBB, left bundle-branch block; LVH, left ventricular hypertrophy; NYHA, New York Heart Association; SaO2, arterial oxygen saturation; SBP, systolic blood pressure; SD, standard deviation.
Data are expressed as No. (%) or mean (standard deviation).
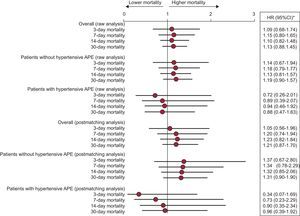
Among the matched patients, there were 264 (19.3%) with hypertensive APE status (135 [19.7%] in the control group and 129 [18.8%] in the nitrates group). The survival analysis of these patients according to hypertensive APE status is shown in Figure 2. Although there were no statistically significant differences, the nitrates group persistently showed HR < 1 compared with the control group when hypertensive APE was present and HR > 1 when hypertensive APE was absent.
Hazard ratio analysis of mortality at 3, 7, 14, and 30 days for patients treated with intravenous nitroglycerin during the acute heart failure episode (nitrates group) compared with patients not receiving nitroglycerin (control group). The analysis is presented for the groups generated after matching, and is presented as both whole and stratified according to whether the patient had hypertensive acute pulmonary edema or not. APE: acute pulmonary edema; HR, hazard ratio; 95%CI, 95% confidence interval. *Compared to the control group (without intravenous nitrates).
The results of the NITRO-EAHFE study can be summarized in 2 relevant findings. First, the study defines the characteristics of patients who received IV nitroglycerin during their care in Spanish hospital ERs. Second, the propensity score analysis showed that the use of IV nitroglycerin does not affect the short-term prognosis of these patients, at least with regard to mortality or new ER visits within 30 days after the index episode. Most likely, in patients with the clinical form of hypertensive APE, nitroglycerin could have beneficial effects in terms of short-term mortality, although this possibility is only suggested by our results and should be confirmed in larger series.
Intravenous nitrates are one of the first-line drugs in the treatment of AHF; however, the level of evidence is IIa, grade of recommendation B, and the effect on early mortality is very poor. Use in Spanish hospital ERs remains steady at 20% to 25% of patients with AHF.16 The NITRO-EAHFE study showed clear differences in patients with AHF treated with IV nitrates compared with the remaining patients. These differences are sometimes due to the protocols at each hospital, but usually depend on the form of presentation and the disease history. Among our patients, those who received IV nitrates had higher cardiovascular risk, younger age, more functional dependence, lower arterial oxygen saturation, faster respiratory rate, faster heart rate, and higher SBP figures. Despite the differences between the 2 groups, no differences were observed in early mortality or readmission in the raw analysis.
Only a few clinical trials have been conducted in hospital ERs, and these studies tend to investigate clinical improvement rather than mortality. Most of these studies did not compare a drug with a placebo, but compared different doses or routes of administration.17,18 Instead, research could be designed to allow the effect of a clinical trial to be simulated, with limitations. Such designs could include stratified analyses, multivariate statistics, and matching techniques based on observational studies.19 The latter have been used in this study with patients included in the EAHFE project; these patients were more heterogeneous in terms of severity than those in published studies with hospitalized patients or in any clinical trials.13 However, the present study has the advantage that it is based on a real population with AHF, which is the population for which the indications of the clinical guidelines were drawn up. The final study included 1370 matched AHF patients who differed only in the use of IV nitrates, a higher number than those included in the clinical trials recently reviewed by the Cochrane Library.11 Both groups had a similar 30-day mortality, which is consistent with the mortality published in the version cited.11 In this case, only 1 study was based on mortality and new visits as the follow-up endpoint,12 although its main objective was hemodynamic and clinical effects in 2 treatment arms (nitroglycerin vs nesiritide). A total of 489 patients were included, and no differences in mortality were found between the 2 groups (0.5% of deaths at 7 days in the nitroglycerin group and 1.5% in the nesiritide group, with an odds ratio for 30-day mortality of 1.91 (95%CI, 0.32-11.52). There were also no significant differences in 30-day readmission (23% in the nitroglycerin group vs 20% in the nesiritide group; P < .36). Although there are limitations when comparing a clinical trial with a registry, the patients in our series who received nitroglycerin therapy had a higher 7-day mortality, a finding most likely related to the sociodemographic differences between the 2 studies, as the clinical trial patients were mainly men and younger than the patients in our population.
The NITRO-EAHFE study performed a separate analysis of the patient subgroup with hypertensive APE. In this case, there were also no significant differences in mortality between the 2 groups, but the HR for mortality (at all time points measured) was below 1. The lack of statistical significance may be influenced by the small number of patients in this situation, which dropped to only 264 overall, as well as by the low number of events due to the lower mortality of patients with hypertensive APE or elevated SBP figures on arrival to the ER20,21 and, therefore, may have led to a beta error. A tendency toward improved mortality was observed among these patients but not among patients who presented with a condition other than hypertensive APE. In 2010, Gray et al13 published a substudy with patients included in the 3CPO clinical trialto determine the effect of diuretics, morphine, and IV nitrates. They found that patients who received IV nitrates had a lower raw mortality. This improvement in 7-day mortality disappeared when the model was adjusted for various confounding factors, such as age or SBP figures at inclusion. This study has limitations because it was a post hoc analysis of a clinical trial intended to measure the influence of noninvasive mechanical ventilation in APE along with the differences between the population included in the trial and the actual population with APE. However, it is one of the few published studies to suggest and, therefore, support the results we have found in the subgroup of patients with APE of the EAHFE registry, namely, that the use of nitrates in this type of patient may have a beneficial effect on mortality, particularly if the patient is in respiratory acidosis.22 Although the scientific evidence is minimal, the results may have important clinical implications: for instance, the early use of these drugs may improve symptoms and reduce mortality in patients with hypertensive APE. These effects have been seen under common health care conditions, but not in a selected population such as that of clinical trials.
LimitationsA limitation of this study is that it did not measure the overall doses of nitroglycerin or identify patients who had to discontinue the drug due to adverse effects. Another limitation is that the study did not measure clinical progress and/or laboratory variables, such as improvements in dyspnea or blood gas parameters. The study also did not consider biological risk markers well contrasted with AHF, eg, troponin,23 natriuretic peptides,24,25 and other more recently introduced markers, such as MR-pro-adrenomedullin or ST2.26,27 Some of these markers are not universally available in Spanish hospital ERs on an emergency basis,28 others are available, but are not routinely requested.23 Lastly, the sample size and the number of events were limited in the estimation of the effect of nitroglycerin on hypertensive APE.
CONCLUSIONSThe NITRO-EAHFE study concludes that IV nitrates do not influence early mortality or new visits in patients with AHF and SBP > 110mmHg. However, the patient subgroup with hypertensive APE showed a positive tendency toward decreased mortality that should be confirmed in future studies.
FUNDINGThis study was conducted as part of the 2008-2011 National R&D Plan projects PI10/01918 and PI11/01021 of the Instituto de Salud Carlos III and was supported by ERDF (European Regional Development Fund) funds. The IDIBAPS (Institut d’Investigacions Biomèdiques August Pi i Sunyer) “Emergency Rooms: Processes and Pathologies” research group was supported by a grant from the Generalitat de Catalunya for consolidated research groups (GRC 2009/1385).
CONFLICTS OF INTERESTNone declared.