We investigated the diagnostic accuracy of technetium-99m-3,3-diphosphono-1,2 propanodicarboxylic acid (99mTc-DPD) scintigraphy in differentiating between monoclonal immunoglobulin light chain and transthyretin-related cardiac amyloidosis.
MethodsNineteen patients with documented cardiac amyloidosis were included: 8 with transthyretin-related amyloidosis (group A) and 11 with light chain amyloidosis (group B). All the patients underwent scintigraphy with 99mTc-DPD and technetium-99m-methylene diphosphonate (99mTc-MDP).
ResultsOn visual scoring, cardiac 99mTc-DPD uptake could be characterized as moderate to severe (scores of 2-3), with ventricular or biventricular distribution, in all group A patients (transthyretin-related cardiac amyloidosis), and was absent or mild (scores of 0-1) and diffusely distributed in all group B patients (monoclonal immunoglobulin light chain cardiac amyloidosis). 99mTc-DPD uptake was also absent (score of 0) among unaffected controls and in 2 unaffected relatives of patients with hereditary transthyretin-related amyloidosis who harbor a mutation in the transthyretin gene. With 99mTc-MDP, all the patients had a myocardial uptake score of 0-1. In our series, selective myocardial uptake of 99mTc-DPD provided 100% accuracy (95% CI, 97.37%-100%) for the differentiation between transthyretin-related and light chain cardiac amyloidosis.
ConclusionsWe conclude that 99mTc-DPD scintigraphy is a useful test for the differential diagnosis of transthyretin versus light chain etiology in patients with cardiac amyloidosis.
Keywords
.
IntroductionCardiac amyloidosis (CA) is caused by the deposition of an insoluble proteinaceous material, amyloid substance, in the cardiac interstitium. The origin and molecular composition of this abnormal protein can vary, thus giving rise to the different types of amyloidosis. Cardiac amyloidosis can form part of a systemic disease and coexist with the involvement of other organs or, more rarely, can involve principally the heart. When it affects the heart, it usually presents as restrictive cardiomyopathy, which leads to death due to heart failure in the majority of the patients.
The various types of CA are currently considered different entities, and distinguishing between them is of great clinical relevance.1
This study began with the incidental finding of a deposit in the myocardium of a patient with severe CA in the context of systemic amyloidosis.2 As a consequence, we decided to evaluate technetium-99m-3,3-diphosphono-1,2 propanodicarboxylic acid (99mTc-DPD) scintigraphy as a useful tool in amyloidosis. This report documents our findings.
MethodsWe studied the utility of 99mTc-DPD scintigraphy in the diagnosis and subtyping of amyloidosis with cardiac involvement.
PatientsThe study population consisted of 19 patients treated for CA in the Heart Failure and Transplantation Section of the Cardiology Service of our center from 1996 to October 2011. All the patients underwent cardiologic evaluation that included electrocardiogram and echocardiogram. The patients evaluated in the final years of the study also underwent cardiac magnetic resonance imaging when it was not contraindicated.
In all the cases, the final diagnosis of amyloidosis was based on the results of a biopsy. If the biopsy was negative in other noncardiac tissues and the clinical suspicion persisted, cardiac biopsy (endomyocardial biopsy) was necessary. In 5 cases, the diagnosis of CA was confirmed by examining the explanted heart following cardiac transplantation. Transthyretin-related cardiac amyloidosis (CA-TTR) was diagnosed in 8 patients (hereditary in 3 and senile in 5) and monoclonal immunoglobulin light chain cardiac amyloidosis (CA-AL) in 11.
We also studied 2 subjects with disease-causing mutations of the TTR gene (Glu89Lys and Val122del, respectively) who were first-degree relatives of patients with confirmed CA and, at the time of the study, had no symptoms of or reason to suspect CA.
As external controls, we included the patients who came to the Nuclear Medicine Service to undergo bone scintigraphy for other purposes (predominantly oncological, including patients with multiple myeloma; they represent a population of over 4000 patients a year), who were randomized to the study with either 99mTc-DPD or technetium-99m-methylene diphosphonate (99mTc-MDP).
Scintigraphic ProtocolThe preparation of the 99mTc-DPD and its quality control were carried out in accordance with the instructions provided by the manufacturer (Teceos®, CIS Bio International; Gif-sur-Yvette, France). The radiochemical purity of the preparation was 98%±1% (97%-99.1%).
Each patient was administered 740 MBq of 99mTc-DPD intravenously, and anterior and posterior whole-body images and selective images in anterior, 45° left anterior oblique, and left lateral projections were obtained 3h later. All of the patients also underwent single photon emission computed tomography (SPECT) of the chest but, given that the images were obtained with different SPECT cameras (4 in all) and using different image acquisition and processing protocols, we preferred not to analyze these images in the present study. The images were obtained using the following parameters: a) whole-body images, LEHR collimator, 512×256 matrix, 3000 kc; and b) selective cardiac images, LEHR collimator, 256×256 matrix, 1000 kc, zoom factor of 1.3.
Two observers from the Nuclear Medicine Service with extensive experience in the evaluation of cardiology studies analyzed the resulting gray-scale images visually. Discrepancies were resolved by consensus.
The results of each examination were assessed, on the one hand, to establish the existence and degree of uptake in the myocardium and, on the other, to determine its distribution. In addition, the ratio between bone uptake and soft tissue uptake of the radiotracer was assessed as an index of adequate clearance and of image quality.
The intensity of the uptake was graded according to a visual scale ranging from 0 to 3 points, in which the absence of uptake was assigned a score of 0 points; uptake less than that of bone (referred to as the adjacent rib), 1 point; uptake similar to that of bone, 2 points; and uptake greater than that of bone, 3 points. The distribution of the uptake in myocardium was defined as focal uptake, diffuse uptake, uptake in a ventricular wall segment, diffuse ventricular uptake, or diffuse biventricular uptake. The ratio of bone uptake to background uptake was assigned a score of 0 when they were very similar and of 1 when the uptake in bone was more or much more marked than in the background.
All of the patients were invited to repeat the study, this time with 99mTc-MDP, but using the same image acquisition protocol and interpretation criteria.
Pathologic StudyThe specimens obtained in a biopsy or from the explanted heart were studied by staining with Congo red and thioflavin to determine the presence of amyloid and by immunohistochemical techniques to determine that of P component, amyloid A, amyloid B, transthyretin, and human immunoglobulin kappa and lambda light chains.
All of the patients with CA-TTR, except patient number 13, underwent a genetic study to differentiate between hereditary and senile TTR amyloidosis.
Statistical AnalysisThe determination of the diagnostic accuracy of the test was based on a 2×2 table designed to establish the proportion of true positives (TP), false positives (FP), true negatives (TN), and false negatives (FN), with the diagnostic accuracy estimators (sensitivity, specificity, and positive and negative predictive values) and their respective confidence intervals, using the appropriate software package (Epidat, v. 3.1).
ResultsThe findings are summarized in the Table 1 and in Figure 1.
Table 1. Results.
| Patient | Sex | Age (Years at Diagnosis) | Type of Amyloidosis | Biopsy Site | 99mTc-DPD | 99mTc-MDP | Monoclonal Paraprotein |
| 1 | Woman | 53 | CA-AL | EM | 0 | 0 | IgM κ |
| 2 | Man | 94 | CA-TTR (s) | EM | 3, BV | 0 | IgG κ * |
| 3 | Woman | 81 | CA-AL | Heart, MSG, liver, kidney | 1, DIF | 0 | IgG λ |
| 4 | Man | 61 | CA-TTR (h) | Heart, lung, CR | 3, BV | 0 | No |
| 5 | Woman | 46 | CA-AL | Heart, EM | 1, DIF | 0 | IgG κ |
| 6 | Woman | 66 | CA-AL | CR | 0 | 0 | IgG λ |
| 7 | Man | 83 | CA-TTR (s) | MSG | 3, BV | 0 | No |
| 8 | Man | 42 | CA-AL | EM, CR, lung | 0 | 0 | IgG λ |
| 9 | Woman | 61 | CA-AL | ABD | 0 | 0 | IgG λ |
| 10 | Man | 53 | CA-TTR (h) | Heart, liver | 3, BV | 0 | No |
| 11 | Man | 55 | CA-AL | Heart, liver | 1, DIF | 0 | IgG λ |
| 12 | Man | 82 | CA-TTR (s) | EM | 3, BV | 0 | No |
| 13 | Woman | 52 | CA-TTR (h) | EM, kidney | 3, BV | 0 | No |
| 14 | Man | 53 | CA-AL | BM | 1, DIF | 1, DIF | IgG λ |
| 15 | Woman | 59 | CA-AL | EM | 1, DIF | 1, DIF | IgG λ |
| 16 | Man | 82 | CA-TTR (s) | EM | 3, BV | 0 | No |
| 17 | Man | 82 | CA-TTR (s) | EM | 3, BV | 0 | No |
| 18 | Man | 53 | CA-AL | EM | 0 | 0 | IgG λ |
| 19 | Woman | 66 | CA-AL | Kidney | 0 | 0 | IgG λ |
ABD, abdomen-peritoneum; BM, bone marrow; BV, biventricular; CA-AL, light chain cardiac amyloidosis; CA-TTR: transthyretin-related cardiac amyloidosis (h: hereditary; s: senile); CR, colorectum; DIF, diffuse; EM, endomyocardium; Heart, explanted heart; MSG, minor salivary gland.
Uptake intensity was graded according to a visual scale ranging from 0 to 3 points, in which the absence of uptake was assigned a value of 0 points; uptake less than that observed in bone (referred to as the adjacent rib), 1 point; uptake similar to that of bone, 2 points; and uptake greater than that of bone, 3 points.
* There was a monoclonal peak of uncertain significance, and bone marrow biopsy ruled out blood dyscrasia.
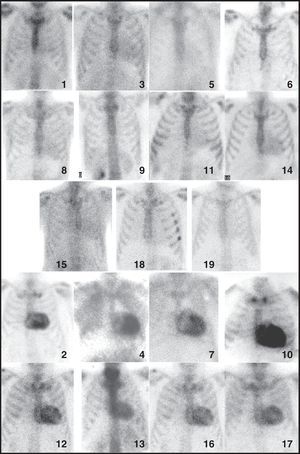
Figure 1. Scintigraphic images showing anterior projection in thorax, obtained with 99mTc-DPD in all the patients in the study. The number corresponds to the patient number in Table.
Neither 99mTc-DPD nor 99mTc-MDP scintigraphy revealed an appreciable deposition in the cardiac region (score of 0) in any of the patients who served as external controls. All of the patients in group A (CA-TTR) had intense 99mTc-DPD uptake (score of 2-3) in the cardiac region, showing deposition in both right and left ventricles in every case (Figure 1, Figure 2). There was no evidence of deposits in the cardiac region in any of the studies performed with 99mTc-MDP (Figure 3).
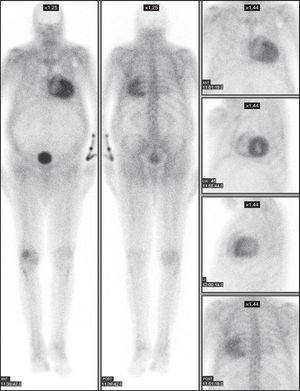
Figure 2. Scintigraphic images in patient no. 7, an 83-year-old man with transthyretin-related cardiac amyloidosis diagnosed by salivary gland biopsy, showing the characteristic biventricular deposition. The distribution of the radiotracer uptake in the myocardial wall of both left and right ventricle can be observed. ANT, anterior; LAO, left anterior oblique; LL, left lateral; POST, posterior.
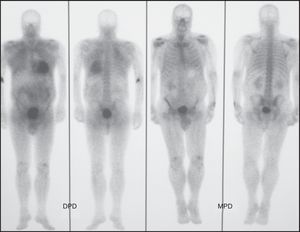
Figure 3. Scintigraphic images in patient no. 4, a 61-year-old man with transthyretin-related cardiac amyloidosis diagnosed by endomyocardial biopsy, showing intense cardiac uptake (grade 3) with 99mTc-DPD and the absence of uptake (grade 0) with 99mTc-MPD. DPD, 3,3-diphosphono-1,2 propanodicarboxylic acid; MDP, methylene diphosphonate.
In 6 patients of group B (CA-AL), there was no appreciable deposition (score of 0) in the cardiac region with 99mTc-DPD, whereas diffuse deposition (score of 1) was observed in 5 patients (Figure 1). The study with 99mTc-MDP evidenced deposition in the cardiac region in only 1 case (patient no. 14); it was diffuse, similar to that observed with 99mTc-DPD, but clearly less intense.
Neither of the 2 subjects without signs of CA who had disease-causing mutations in the TTR gene (relatives of patients with TTR-related hereditary CA) showed myocardial 99mTc-DPD uptake.
The analysis of the results obtained revealed a moderate to intense 99mTc-DPD uptake (score of 2-3) in both ventricles, a finding that corresponded to a sensitivity of 100% (95% confidence interval [CI], 93.75%-100%), a specificity of 100% (95% CI, 95.45%-100%), a positive predictive value of 100% (95% CI, 93.75%-100%), a negative predictive value of 100% (95% CI, 95.45%-100%), and a diagnostic accuracy (overall value of the test) of 1 (100%) (95% CI, 97.37%-100%) in the detection of patients with CA-TTR; moreover, the observed agreement was 100% and the kappa coefficient, +1.
The interobserver and intraobserver agreements between the 2 experts in nuclear medicine who evaluated the studies (FJHM and ASL) were 100%.
DiscussionThe amyloidoses constitute a heterogeneous group of diseases involving the extracellular deposition of insoluble abnormal fibrils made up of different low molecular weight subunits (between 5 kDa and 25 kDa). Pathological examination reveals that the amyloid deposits appear as hyaline material that stains with different dyes such as Congo red (showing green birefringence under polarized light), thioflavin T (which produces an intense yellowish-green fluorescence), and Alcian blue (producing a green stain).
The amyloidoses can be classified as systemic or localized, depending on whether the deposits are found in several organs, vascular walls, and connective tissue, or are confined to a single organ or tissue.3
Although several types of amyloid can infiltrate the heart, only some of the more than 20 known amyloidotic proteins produce cardiac involvement. These correspond to the senile, secondary (AA), and primary (AL) variants, and to certain hereditary forms (such as ATTR, AApoA-I, and AFib); the most common forms are CA-AL and CA-TTR (either hereditary or senile).4
The clinical features of cardiac involvement are similar in all of these variants and are determined by the infiltrative pattern, which affects contractile function as well as vascular flow and conduction. Thus, the clinical signs of heart disease are mainly diastolic heart failure, angina, syncope, presyncope, and arrhythmias.5
Each type of amyloidosis with cardiac involvement represents a separate entity as their course, treatment, and prognosis differ and, in those cases in which the disease is due to a genetic defect, the diagnosis is highly important for the relatives of the patient.6, 7 For this reason, it is essential to determine the subtype of amyloidosis of each and every patient.
Traditionally, echocardiography has been considered the method of choice for the noninvasive diagnosis of amyloidotic cardiomyopathy.8, 9 In recent years, cardiac magnetic resonance imaging is being employed with increasing frequency for the evaluation of cardiac involvement in these patients.10, 11, 12, 13 However, neither of these imaging techniques makes it possible to determine the composition of amyloid deposits, which can only be distinguished in the histopathological study.10
The diagnostic confirmation of CA requires the demonstration of the deposition of amyloid in a biopsy, though it does not necessarily have to be in heart tissue if there are other organs involved (provided there is evidence of cardiac involvement in the imaging studies); however, it is the cardiac biopsy that ensures a sensitivity of 100% for the detection of the disease.14
The noninvasive detection of cardiac involvement in the course of amyloidosis has been investigated using a number of radiotracers.15 Research has been based on the identification of changes in different physiological mechanisms due to the myocardial infiltration, such as alterations in the innervation, in myocardial metabolism or perfusion, myocyte membrane damage, or changes in ventricular function.
However, for the detection of amyloid deposits, basically 3 groups of radiotracers have been used: 123I-labeled serum amyloid P component (SAP), 99mTc-labeled aprotinin, and 99mTc-labeled bone tracers; those most widely used are 99mTc-pyrophosphate (99mTc-PYP) and 99mTc-MDP.
Given that labeled SAP is not commercially available (with the exception of a few centers in the entire world), that its sensitivity in patients with TTR amyloidosis is poor, and that, in addition, it does not enable the characterization of the deposits in dynamic structures like the heart, its utility in CA is very limited.16, 17, 18
The results with 99mTc-labeled aprotinin have been promising, but there is little experience with this tracer and an important limitation to its use is the fact that follow-up of the amount of deposition present is not possible, especially in myocardium.15, 19, 20, 21
Scintigraphy with phosphonate compounds has been used for years for the detection of myocardial injury. Over the last three decades, the uptake of 99mTc-labeled bone radiotracers in CA has been extensively documented, both in CA-AL and in CA-TTR,22, 23, 24, 25, 26, 27 with relatively discrepant results and a sensitivity and specificity subject to a wide debate.
It can be said that the publication of the article by Puille et al. in 200228 initiates a new era in the scintigraphic detection of amyloid deposition with the use of 99mTc-DPD in patients with CA-TTR. Since that pioneering work there have been a number of publications related to the subject, and Peruggini et al.29 even proposed 99mTc-DPD scintigraphy as an interesting technique for distinguishing between CA-TTR and CA-AL cardiac deposits. According to these authors, 99mTc-DPD uptake would be detected in the hearts of patients with CA-TTR (whether hereditary or senile), whereas it would be absent in the hearts of patients with CA-AL. Other authors, such as Selvanayagam et al.,30 do not share this hypothesis and point out the few published cases and the lack of evidence of the existence of a molecular mechanism that enables binding between bone tracers and the amyloid deposit, a circumstance that, in the light of what is known to date, suggests a certain degree of inconsistency.31, 32
After employing this technique for several years, in the present study we found that the selective myocardial uptake of 99mTc-DPD has an accuracy of 100% for the differentiation between CA-TTR and CA-AL, which corroborates the proposal of Peruggini et al.29 Our report also shows that when the uptake is intense or absent it allows us to discriminate effectively between the two etiologies, but nearly half of the subjects with AL amyloidosis (45%, 5 of 11 patients) exhibit mild cardiac uptake (a score of 1). This mild uptake could be explained by the persistence of the radiotracer in the bloodstream (vascular pool) secondary to the limited clearance produced by heart failure and fluid retention, rather than by a true deposit in the myocardium. The observation of similar findings, although less intense, in the image obtained with 99mTc-MDP in one of our patients (Figure 3, patient no. 4) soon after administration of the radiotracer and the disappearance of the deposit later on (Figure 4, patient no. 14) would indicate the nonspecificity of said uptake.
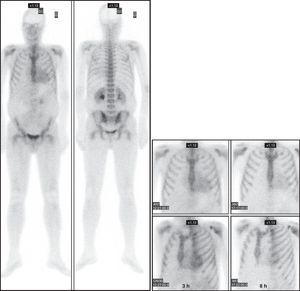
Figure 4. Scintigraphic images in patient no. 14, a 46-year-old man with monoclonal immunoglobulin light chain cardiac amyloidosis, showing mild, diffuse cardiac uptake (grade 1) soon after radiotracer administration. It can be seen to disappear (grade 0) with the passage of time. ANT, anterior; LAO, left anterior oblique.
On the other hand, it is important to stress the fact that the mere existence of a cardiac amyloid deposit detected with 99mTc-DPD does not lead to the diagnosis, which is based on the intensity and distribution of the uptake in the cardiac wall; that is to say, it is the observation of intense uptake in both ventricles (Figure 1), or at least in one, that is of diagnostic value.
The intimate mechanism of the binding of the bisphosphonate to amyloid has not been defined. The earliest studies indicated the existence of high concentrations of calcium in the amyloidotic tissue, and it has been suggested that the radiotracer, with its high avidity for calcium, would accumulate in the amyloid, much like what occurs in calcifications.33 It has also been postulated that the amount of amyloid deposited in the myocardium could be the cause of the presence or absence of bisphosphonate uptake. This idea was refuted by the observation of the absence of a relationship between myocardial mass and the presence of deposition in both echocardiographic studies and those performed with radiotracers.29
The apparent preference of 99mTc-DPD for CA-TTR compared to CA-AL in cardiac involvement remains a mystery. Our study shows that 99mTc-DPD uptake was observed in all the patients with CA-TTR, where none of them exhibited 99mTc-MDP uptake. A number of researchers defend the possibility that this could be due to an especially high calcium concentration in the myocardium in this amyloidosis subgroup, secondary to the different composition of the amyloid fibrils, and that these would have different affinities for bisphosphonates.34 This hypothesis would explain the mixed results historically obtained with the different phosphonates employed (MDP, HDP, PYP) both in the diagnosis of the disease and in the differentiation among the types of amyloidosis.
One aspect that we wish to point out and that we find interesting is the fact that the 2 subjects we studied who had relatives with documented hereditary CA-TTR did not have myocardial deposition, although they did have disease-causing mutations in the TTR gene. Given that these 2 subjects showed no evidence of cardiac involvement, this finding may indicate that in order for there to be uptake on scintigraphy there must be significant deposition of amyloid in the myocardium.
As CA is an uncommon disease that is difficult to recognize, most of the published reports involve small numbers of cases. Our study also has this limitation, since the number of cases of CA documented in a single center is always low, even though the inclusion of patients continues over a long period of time. Only the report recently published by Rapezzi et al.34 deals with a considerable number of cases, and not single cases as occurs in most of the publications on the subject. In contrast to other studies, in ours the type of amyloidosis is clearly documented by pathological examinations. We feel that, by adding more cases to the literature, we help to clarify the confusing scenarios to which publications that report a single case and/or do not specify the type of CA being studied have contributed, a situation that is the basis, and rightly so, for the criticisms voiced by, among others, the group of Selvanayagam.32
ConclusionsCardiac scintigraphy with 99mTc-DPD is a useful tool for the differential diagnosis of CA-TTR and CA-AL in patients with documented cardiac CA. In these patients, 99mTc-DPD scintigraphy showing intense cardiac uptake in both ventricles indicates a diagnosis of CA-TTR, whereas a negative 99mTc-DPD scintigram rules it out.
Conflicts of interestNone declared.
Received 18 October 2011
Accepted 11 December 2011
Corresponding author: Servicio de Medicina Nuclear, Hospital Universitario Puerta de Hierro, Manuel de Falla 2, 28222 Majadahonda, Madrid, Spain. fjdeharo.hpth@salud.madrid.org