Percutaneous closure of paravalvular leakage is an alternative to surgery in high-risk patients, but its use has been limited by a lack of specific devices. More appropriate devices—like the Amplatzer Vascular Plug III—have recently been developed, but information about their efficacy and safety is still scarce. The objective of the present study was to assess the mid-term results of paravalvular leakage closure with this device.
MethodsWe analyzed the clinical and echocardiographic course both in-hospital and mid-term (13 [9] months) in a series of 20 consecutive patients (age, 68 years; logistic EuroSCORE, 29) with paravalvular leakage and attempted percutaneous closure.
ResultsClosure was attempted for 23 leaks (17 mitral and 6 aortic) during 22 procedures in 20 patients. Implantation was successful in 87% of the leaks and the procedure was successful in 83%—with success being defined as a reduction in regurgitation of ≥ 1 degree. Survival at 1 year was 64.7% and survival free of the composite event of death/surgery was 58.8%. The degree of residual regurgitation was not associated with mortality but was associated with functional status. Survivors showed significant improvement in functional class.
ConclusionsPercutaneous closure of leakage with the Amplatzer Vascular Plug III is safe and efficient in the mid-term. However, mortality among high-risk patients is high independently of the degree of residual regurgitation, indicating that these procedures are performed when heart disease has reached an advanced stage.
Keywords
Paravalvular leakage (PVL) is a frequent complication after the implantation of a prosthetic valve. This complication is due to failure of the surgical suture, favored by the presence of calcium, infection, tissue friability, or the noncircular shape of the annulus.1,2 In most cases, PVL is small, being found by chance during postsurgical echocardiography. Only 1% to 5% of PVL causes symptoms of either congestive heart failure (in patients with large PVL), or hemolytic anemia (in smaller, tortuous, and multiple PVL).2
Medical treatment can improve symptoms but cannot correct the structural defect and consequently the treatment of choice has traditionally been surgical reoperation to close the defect and/or replace the prosthesis.1 However, given the underlying predisposing factors, reoperation is associated with higher morbidity and mortality than initial interventions and is also associated with a higher rate of residual or recurrent PVL.3
Recently, percutaneous closure has been proposed as an alternative to surgery in high-risk patients, especially due to the development of devices with designs better–suited to PVL closure, like the Amplatzer Vascular Plug (AVP) III (AGA, St. Jude Medical; Minneapolis, Minnesota, United States). However, the few reports published on this device are limited to the immediate results of isolated cases and small series and no studies have assessed the long-term results. The objective of the present study was to analyze the immediate and mid-term clinical and echocardiographic course of a series of consecutive patients treated with the AVP III device as first choice.
METHODSStudy PopulationFrom September 2010 to September 2012, percutaneous closure was scheduled in 22 patients in a single center. All were symptomatic and attended special sessions with participation by clinical cardiologists, interventional cardiologists, and surgeons. Two patients were excluded from percutaneous closure: 1 had an infectious aortic pseudoaneurysm with detachment of the prosthesis; the other had detachment of a mitral annulus (with no prosthesis) affecting more than one third of the circumference. All patients signed consent forms explaining the risks and benefits of this off-label use of the AVP III device.
Hemolytic anemia was defined as hemoglobin ≥ 10g/dL and hemolysis requiring blood transfusion. Heart failure was defined as dyspnea in New York Heart Association (NYHA) functional class > II. Technical success was defined as implantation of the device at the PVL that did not interfere with normal functioning of the prosthesis or require urgent surgery. Procedural success was defined as technical success with a reduction in paravalvular regurgitation of ≥ 1 degree.
Percutaneous Closure TechniqueAll interventions were guided by 3-dimensional transesophageal echocardiography with the patient intubated. Intravenous heparin was administered after transseptal puncture and cefazolin was administered as prophylaxis. In all cases of aortic PVL, an aortogram was used to locate the PVL and retrograde access was used for the implantation. A 0.035″ hydrophilic guidewire was passed from the aorta to the left ventricle with the help of a 5-Fr diagnostic coronary catheter, curved to match the PVL site. With the guidewire in the left ventricle, the 5-Fr diagnostic catheter was introduced and the hydrophilic guidewire was replaced by a 0.035″ high support guidewire. Finally, an appropriately-sized device release sheath was advanced along the guidewire; the guidewire was withdrawn; and, through the delivery sheath, the device was deployed at the PVL.
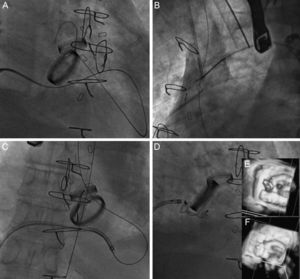
In mitral PVL, the standard approach was anterograde; a transseptal puncture was made to position a Mullins sheath in the left atrial area. A 5-Fr coronary catheter with a 0.035″ hydrophilic guidewire was advanced through the sheath in an attempt to pass the guidewire from the atrium to the left ventricle and aorta. At this level, with a snare, the guidewire was externalized through the femoral artery and an arteriovenous loop was created. Finally, the device release sheath was advanced through the guidewire from the venous side to implant the device. In 3 cases, an Agilis steerable catheter (St. Jude Medical) was used to maneuver the guidewire in the left atrial area toward the PVL. In 2 cases, the approach was retrograde, with the PVL being passed from the left ventricle to the left atrial area; a snare was used at the level of a pulmonary vein to entrap the guidewire for its externalization and to create an arteriovenous loop. Of the patients with mitral PVL, 8 also had mechanical aortic prosthesis implants. In all of these patients, an arteriovenous loop was created, using a hydrophilic guidewire (Terumo; Tokyo, Japan), due to the lack of support to advance the release sheath. In bileaflet prosthetic aortic valves, the guidewire was passed through one of the lateral orifices; in monoleaflet prostheses, the guidewire was passed through the main orifice. In 1 patient with mitral PVL and aortic PVL, the guidewire was passed through the aortic defect, thus avoiding the inside of the mechanical prosthesis. An example is shown in Figure 1.
Closure of posterior paravalvular leakage on a mechanical mitral valve in the presence of bileaflet mechanical aortic prosthesis. A: the hydrophilic guidewire is advanced by anterograde access through the mitral paravalvular leak and into the aortic prosthesis. B: the guidewire is entrapped in the ascending aorta with a snare and externalized through a femoral artery to create an arteriovenous loop. C: this gives us sufficient push for the release sheath to pass. D: an Amplatzer Vascular Plug III 14/5 device is implanted. E: baseline 3-dimensional transesophageal echocardiography image. F: result postimplantation.
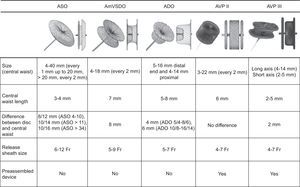
The first option was to implant an AVP III device, although in some cases an Amplatzer Duct Occluder or an Amplatzer Ventricular Septal Occluder was finally used due to the morphology, PVL size, or interference of the AVP III with a disc in the prosthesis. The characteristics of the device types are shown in Figure 2. When choosing device size, we took account of the largest and smallest diameters of the defect measured with 3-dimensional transesophageal echocardiography reconstruction, with and without color. We used a device equal to, or 1 to 2mm larger than the reference defect diameters in the echocardiogram. In large PVL, we used 2 AVP III simultaneously, 1 Amplatzer Duct Occluder II, or 1 Amplatzer Ventricular Septal Occluder.
Characteristics of devices traditionally used in paravalvular leakage closure, all from St. Jude Medical (St. Paul, Minnesota, United States). ADO, Amplatzer Duct Occluder; AmVSDO, Amplatzer Ventricular Septal Defect Occluder; ASO, Amplatzer Septal Occluder; AVP, Amplatzer Vascular Plug.
Data were recorded on the procedure and on in-hospital complications, such as death or urgent procedure-related surgery, cardiovascular death and all-cause death, neurologic events, vascular complications requiring surgery or blood transfusion, cardiac tamponade or myocardial infarction.
Clinical and Echocardiographic Follow-upPatients were followed up in a dedicated clinic at 1, 6 and 12 months and subsequently in annual visits. During follow-up, patients underwent ≥ 1 transthoracic echocardiographic study; those with a mitral prosthesis also underwent a transesophageal study. We collected clinical data on functional class, need for transfusion, cardiovascular and all-cause deaths, and neurologic and cardiac events. Echocardiographic studies provided data on ventricular function, pulmonary pressure, and the degree (on a 0-4 scale) of semiquantitative (mild, moderate and severe) and quantitative residual regurgitation.
Statistical AnalysisQualitative variables are expressed as number and percentage. Quantitative variables are presented as mean (standard deviation). Event-free survival was analyzed by constructing Kaplan-Meier curves. The nonparametric Mann-Whitney test was used to compare the EuroSCORE of the first and last 10 patients. The Wilcoxon test was used to compare pulmonary pressure, ejection fraction, and pre- and postoperative functional class according to the degree of residual regurgitation (classified as binary: 0-2 and 3-4). The chi-square test was used to study the relation between the degree of residual regurgitation and mortality and functional class. The analysis was performed with SPSS 21.0. (SPSS, Inc.; Chicago, Illinois, United States).
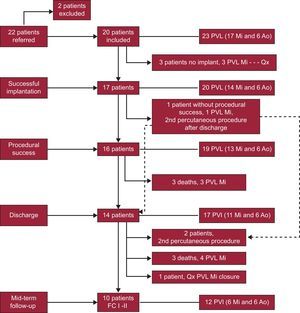
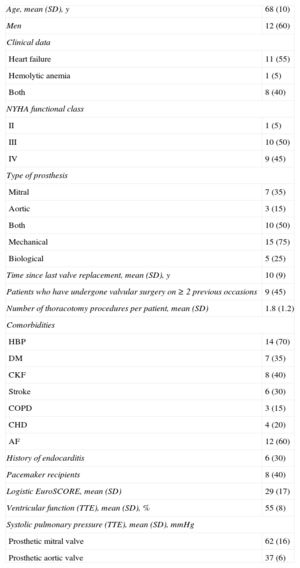
RESULTSPercutaneous closure was performed in 23 PVL (17 mitral and 6 aortic) in 20 patients in 22 procedures. Baseline clinical characteristics are shown in Table 1. One patient underwent percutaneous closure of a mitral PVL and an aortic PVL in the same procedure; 2 patients required 2 separate procedures to achieve complete PVL closure. Importantly, this population had high comorbidity and very high surgical risk (mean logistic EuroSCORE, 29 [17]). Dividing the sample at random and comparing the EuroSCORE between the first and the last 10 patients revealed that the mean logistic EuroSCORE was 39 (20) for the first 10 patients and 19 (11) for the last 10 (P=.01). This finding indicates that the PVL percutaneous closure program was initiated with patients at very high surgical risk.
Demographic and Baseline Clinical Characteristics of the 20 Patients
| Age, mean (SD), y | 68 (10) |
| Men | 12 (60) |
| Clinical data | |
| Heart failure | 11 (55) |
| Hemolytic anemia | 1 (5) |
| Both | 8 (40) |
| NYHA functional class | |
| II | 1 (5) |
| III | 10 (50) |
| IV | 9 (45) |
| Type of prosthesis | |
| Mitral | 7 (35) |
| Aortic | 3 (15) |
| Both | 10 (50) |
| Mechanical | 15 (75) |
| Biological | 5 (25) |
| Time since last valve replacement, mean (SD), y | 10 (9) |
| Patients who have undergone valvular surgery on ≥ 2 previous occasions | 9 (45) |
| Number of thoracotomy procedures per patient, mean (SD) | 1.8 (1.2) |
| Comorbidities | |
| HBP | 14 (70) |
| DM | 7 (35) |
| CKF | 8 (40) |
| Stroke | 6 (30) |
| COPD | 3 (15) |
| CHD | 4 (20) |
| AF | 12 (60) |
| History of endocarditis | 6 (30) |
| Pacemaker recipients | 8 (40) |
| Logistic EuroSCORE, mean (SD) | 29 (17) |
| Ventricular function (TTE), mean (SD), % | 55 (8) |
| Systolic pulmonary pressure (TTE), mean (SD), mmHg | |
| Prosthetic mitral valve | 62 (16) |
| Prosthetic aortic valve | 37 (6) |
AF, atrial fibrillation; CHD, coronary heart disease; CKF, chronic kidney failure; COPD, chronic obstructive pulmonary disease; DM, diabetes mellitus; HBP, high blood pressure; NYHA, New York Heart Association; SD, standard deviation; TTE, transthoracic echocardiography.
Data are expressed as n (%) or mean (standard deviation).
Of the 23 PVL in 20 patients with attempted percutaneous closure, implantation was successful in 87% (85% of patients) and the procedure was successful in 83% (80% of patients). Closure was not achieved in 3 mitral PVL in 3 patients with double mechanical mitral and aortic prostheses. In 1 patient, we were unable to pass the guidewire through the defect in a tortuous mitral PVL in a septal site, by the anterograde or retrograde approach, despite using a deflectable, steerable catheter like the Agilis. In another patient, the guidewire passed but the delivery sheath did not, despite the creation of an arteriovenous loop. In the third patient, an AVP III device was successfully implanted after we created an arteriovenous loop. However, the device constantly interfered with the mitral disc, despite several attempts at repositioning. All 3 patients were indicated for surgery.
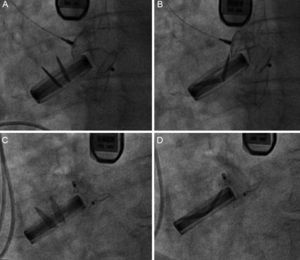
Finally, 21 Amplatzer devices were implanted in the 20 PVL (17 patients) successfully closed. One patient received 2 devices simultaneously, due to a large PVL. Eighteen AVP III devices (86%) were implanted, as their morphology was better suited to the defect. Two Amplatzer Duct Occluder and 1 Amplatzer Ventricular Septal Occluder devices were also implanted. In 1 patient with an aortic PVL and an AVP III implant, we found that the device interfered with a disc in the aortic prosthesis and an Amplatzer Duct Occluder device was substituted to avoid this complication (Figure 3). Procedure success was achieved in 83% of the PVL: 100% of the aortic PVL and 77% of the mitral PVL.
Amplatzer Vascular Plug III 12/5 device implant in aortic position. A: in systole, the device does not interfere with the prosthesis. B: in diastole, the device does interfere with the prosthesis. C: an Amplatzer Duct Occluder 12/10 device implant that does not interfere in systole. D: or in diastole.
The in-hospital complications recorded were as follows: there were 3 deaths; of these, only 1 was procedure-related in a patient who, after successful closure of a mitral PVL, had bleeding from the femoral artery with anemia requiring surgical repair for a small rupture of the femoral artery and, at 48h, developed signs and symptoms of fatal intestinal ischemia. In the other 2 patients, percutaneous closure was performed in the context of end-stage heart failure and, despite procedural success, the outcome was fatal. One patient was already intubated for acute pulmonary edema and it was impossible to proceed with extubation postprocedure. The patient died of multiple organ failure, even though mitral regurgitation was no more than mild at 20 days. The other patient also had mild-moderate mitral regurgitation after closure but died of a respiratory infection and septic shock at 15 days. Furthermore, in another patient, pseudoaneurysms formed in both femoral arteries, despite the use of a percutaneous closure device and the absence of complications during the intervention. There were no cases of device embolization or interference with normal prosthesis function requiring urgent surgery. Data on procedures and complications are shown in Table 2. A flow-chart of the clinical course of all patients is provided in Figure 4.
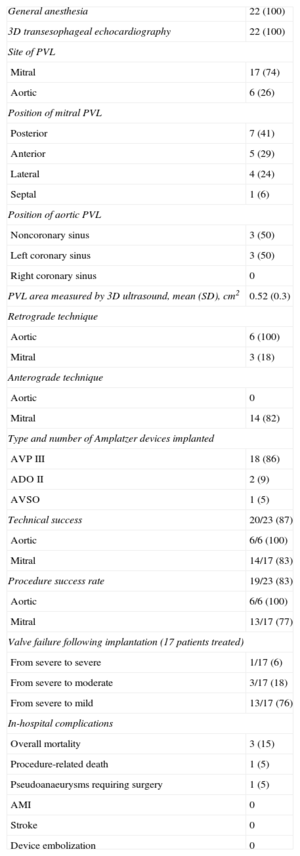
Results and Immediate Complications of the 22 Procedures for Closure of 23 Paravalvular Leaks
| General anesthesia | 22 (100) |
| 3D transesophageal echocardiography | 22 (100) |
| Site of PVL | |
| Mitral | 17 (74) |
| Aortic | 6 (26) |
| Position of mitral PVL | |
| Posterior | 7 (41) |
| Anterior | 5 (29) |
| Lateral | 4 (24) |
| Septal | 1 (6) |
| Position of aortic PVL | |
| Noncoronary sinus | 3 (50) |
| Left coronary sinus | 3 (50) |
| Right coronary sinus | 0 |
| PVL area measured by 3D ultrasound, mean (SD), cm2 | 0.52 (0.3) |
| Retrograde technique | |
| Aortic | 6 (100) |
| Mitral | 3 (18) |
| Anterograde technique | |
| Aortic | 0 |
| Mitral | 14 (82) |
| Type and number of Amplatzer devices implanted | |
| AVP III | 18 (86) |
| ADO II | 2 (9) |
| AVSO | 1 (5) |
| Technical success | 20/23 (87) |
| Aortic | 6/6 (100) |
| Mitral | 14/17 (83) |
| Procedure success rate | 19/23 (83) |
| Aortic | 6/6 (100) |
| Mitral | 13/17 (77) |
| Valve failure following implantation (17 patients treated) | |
| From severe to severe | 1/17 (6) |
| From severe to moderate | 3/17 (18) |
| From severe to mild | 13/17 (76) |
| In-hospital complications | |
| Overall mortality | 3 (15) |
| Procedure-related death | 1 (5) |
| Pseudoanaeurysms requiring surgery | 1 (5) |
| AMI | 0 |
| Stroke | 0 |
| Device embolization | 0 |
3D, 3-dimensional; ADO, Amplatzer Duct Occluder; AMI, acute myocardial infarction; AVP, Amplatzer Vascular Plug; AVSO, Amplatzer Ventricular Septal Occluder; PVL, paravalvular leakage; SD, standard deviation.
Data are expressed as n/N (%) or mean (standard deviation).
Flow chart of patients by implant success, procedure success, discharge and long-term follow-up. The broken arrow indicates a patient implanted successfully but with continued severe mitral regurgitation and successful defect closure in a second procedure after discharge. Ao, aortic; FC, functional class; Mi, mitral; PVL: paravalvular leakage; Qx, paravalvular leakage closure surgery.
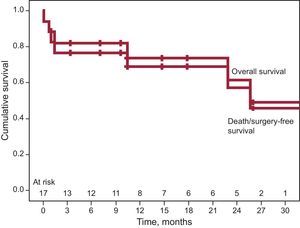
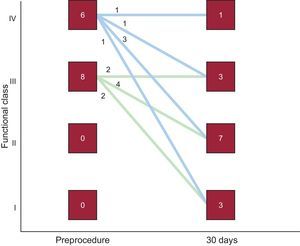
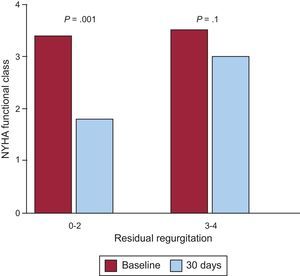
After a mean follow-up of 13 (9) (median, 12) months, 3 of the 14 patients discharged with moderate residual mitral regurgitation and in NYHA III, died—2 of end-stage heart failure. The third patient had mild regurgitation after a second procedure and was in NYHA I, finally dying of breast cancer. One patient required surgery after refusing to undergo a second percutaneous procedure, given that he or she was in NYHA III, required periodic transfusions, and had severe residual mitral regurgitation. Hence, long-term cumulative survival was 64.7% and survival free of the composite event of death/surgery was 58.8%. Kaplan-Meier curves are shown in Figure 5. The degree of residual valvular regurgitation was not significantly related to mortality. Of the 14 patients discharged, 72% showed improvement in functional class at 30 days postprocedure—except the 4 patients previously mentioned (Figure 6). We confirmed a significant relation between the degree of residual valvular regurgitation and NYHA functional class at 30 days. Functional class significantly improved in patients with residual regurgitation ≤ 2 but not in those with residual regurgitation >2 (Figure 7). In the long-term, the 10 patients who survived and were without surgery were found to be in NYHA I-II, which demonstrates a significant improvement in functional class (P=.001). The echocardiographic parameters showed a significant improvement in residual valvular regurgitation, which was mild in 9 patients and moderate in 1 patient who had an aortic PVL but was in NYHA II (P=.001). Estimated systolic pulmonary pressure decreased from 56 (16) mmHg to 48 (10) mmHg (P=.04) after percutaneous closure. No significant changes were found in left ventricular ejection fraction.
The major findings of the present study are that percutaneous PVL closure with the AVP III device is a feasible procedure, with high technical and procedural success rates. Secondly, long-term mortality was high in a cohort of high-risk patients, with no relation to residual valvular regurgitation, indicating that percutaneous closure is performed at an advanced stage of the illness. Finally, survivors showed a clear long-term improvement in functional class, related to the degree of residual valvular regurgitation.
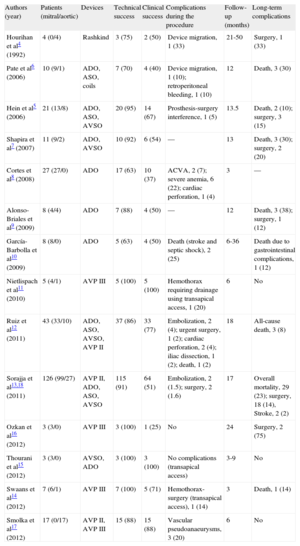
Percutaneous closure of PVL was developed 20 years ago as an alternative to surgery in high-risk patients.4 Initially performed in certain specific centers, the technique has been perfected in recent years and its use has spread. This has coincided with the development of vascular and intracardiac defect closure devices, although none was specifically designed for PVL. Isolated clinical cases and short series show that this is a complex but feasible technique, with a high procedure success rate. Table 3 shows all published series of percutaneous closure of PVL with ≥ 3 patients, irrespective of the use of transfemoral or transapical access.4–18 The technical success rate of all the series ranged from 63% to 100%. In the 2 larger series,12,13,18 which are relatively recent, the technical success rate was 86% and 89%. This rate indicates that, although PVL closure is a complex procedure—especially in the mitral position—the sum total of experience gathered in recent years has made device implantation in PVL feasible and successful in a high percentage of cases. The off-label use of devices like the AVP III, with a design better-suited for PVL closure, could hypothetically increase procedural success. Experience with this new device is limited to publications on isolated clinical cases and very short patient series.11,14,16,17 In 3 series, transapical access was systematically used for closure of mitral PVL,11,14,16 and another series also included patients treated with AVP II.17 Our series is one of the widest-ranging of patients treated with AVP III through transfemoral access and with ≥ 1 year of follow-up. The rates of technical and procedural success were 87% and 83%—comparable to those of other recent series using other devices.12,13,18 The AVP III is limited in terms of size. Hence, it is impossible to treat PVL >14mm with a single device; moreover, one of the discs that extends beyond the waist can occasionally block the prosthesis, as shown in Figure 3. However, the AVP III undoubtedly represents a further qualitative improvement by comparison with previously used devices. Its oval morphology adapts better to PVL anatomy, it comes preassembled, has a better crossing profile, and can be delivered through smaller caliber sheaths, which means it crosses the PVL more easily. Nonetheless, despite the improved devices, technical and procedural success rates remain little changed; the reasons for this lack of change are closure of some mitral PVL, larger PVL, small PVL with serpentine trajectories, multiple PVL, PVL in lateral or septal sites, enlargement of small PVL when forcing the sheath to advance, and the appearance of new contralateral PVL after closure.
Studies with Series of Percutaneous Closure of Paravalvular Leakage (N≥3 patients)
| Authors (year) | Patients (mitral/aortic) | Devices | Technical success | Clinical success | Complications during the procedure | Follow-up (months) | Long-term complications |
| Hourihan et al4 (1992) | 4 (0/4) | Rashkind | 3 (75) | 2 (50) | Device migration, 1 (33) | 21-50 | Surgery, 1 (33) |
| Pate et al6 (2006) | 10 (9/1) | ADO, ASO, coils | 7 (70) | 4 (40) | Device migration, 1 (10); retroperitoneal bleeding, 1 (10) | 12 | Death, 3 (30) |
| Hein et al5 (2006) | 21 (13/8) | ADO, ASO, AVSO | 20 (95) | 14 (67) | Prosthesis-surgery interference, 1 (5) | 13.5 | Death, 2 (10); surgery, 3 (15) |
| Shapira et al7 (2007) | 11 (9/2) | ADO, AVSO | 10 (92) | 6 (54) | — | 13 | Death, 3 (30); surgery, 2 (20) |
| Cortes et al8 (2008) | 27 (27/0) | ADO | 17 (63) | 10 (37) | ACVA, 2 (7); severe anemia, 6 (22); cardiac perforation, 1 (4) | 3 | — |
| Alonso-Briales et al9 (2009) | 8 (4/4) | ADO | 7 (88) | 4 (50) | — | 12 | Death, 3 (38); surgery, 1 (12) |
| García-Barbolla et al10 (2009) | 8 (8/0) | ADO | 5 (63) | 4 (50) | Death (stroke and septic shock), 2 (25) | 6-36 | Death due to gastrointestinal complications, 1 (12) |
| Nietlispach et al11 (2010) | 5 (4/1) | AVP III | 5 (100) | 5 (100) | Hemothorax requiring drainage using transapical access, 1 (20) | 6 | No |
| Ruiz et al12 (2011) | 43 (33/10) | ADO, ASO, AVSO, AVP II | 37 (86) | 33 (77) | Embolization, 2 (4); urgent surgery, 1 (2); cardiac perforation, 2 (4); iliac dissection, 1 (2); death, 1 (2) | 18 | All-cause death, 3 (8) |
| Sorajja et al13,18 (2011) | 126 (99/27) | AVP II, ADO, ASO, AVSO | 115 (91) | 64 (51) | Embolization, 2 (1.5); surgery, 2 (1.6) | 17 | Overall mortality, 29 (23); surgery, 18 (14), Stroke, 2 (2) |
| Ozkan et al16 (2012) | 3 (3/0) | AVP III | 3 (100) | 1 (25) | No | 24 | Surgery, 2 (75) |
| Thourani et al15 (2012) | 3 (3/0) | AVSO, ADO | 3 (100) | 3 (100) | No complications (transapical access) | 3-9 | No |
| Swaans et al14 (2012) | 7 (6/1) | AVP III | 7 (100) | 5 (71) | Hemothorax-surgery (transapical access), 1 (14) | 3 | Death, 1 (14) |
| Smolka et al17 (2012) | 17 (0/17) | AVP II, AVP III | 15 (88) | 15 (88) | Vascular pseudoanaeurysms, 3 (20) | 6 | No |
ADO, Amplatzer Duct Occluder; ASO, Amplatzer Septal Occluder; AVP, Amplatzer Vascular Plug; AVSO, Amplatzer Ventricular Septal Occluder.
Unless otherwise indicated, values expressed as n (%).
Furthermore, mitral PVL closure in the presence of a mechanical aortic prosthesis, although feasible, is not without difficulties.19 In our series, no major complications resulted from the creation of an arteriovenous loop, but hemodynamic deterioration did occur, requiring the mobilization (movements to tense and free) of the hydrophilic guidewire to recover movement of a blocked disc. This complication demonstrates the importance of using echocardiography, fluoroscopy, and invasive pressure to guide the procedure. Transapical access, which was not used in our series, could avoid these technical problems and increase the technical success rate.20,21
The percentage of complications varies greatly in the literature. Those most frequently reported are vascular complications, urgent surgery for device embolization or interference with the prosthesis, perioperative stroke and bleeding (Table 3). Procedure-related death is infrequent in published series—as it was in ours—leading to the conclusion that despite its complexity, the procedure is safe.
All-cause mortality in previous studies with ≥ 1 year follow-up ranged from 8% to 38%.4,7,9,10,12,13 In a study by Sorajja et al,13 including the largest series and with a mean follow-up of 17 months, mortality was 23% and the most frequent cause of death was end-stage heart failure. Our series was composed of very high-risk patients with high comorbidity, as indicated by their logistic EuroSCORE of 29. Mortality was 30% in a mean follow-up of 1 year and 66% of deaths were caused by end-stage heart failure. There was no relation between the degree of residual valvular regurgitation and mortality. This finding suggests that, in patients rejected for surgery, percutaneous closure was indicated when the disease was in an advanced stage, leading to a fatal outcome in the mid-term, independently of the degree of valvular regurgitation. Therefore, we believe that PVL closure should be indicated at an earlier stage in the clinical course of valvular heart disease rather than waiting for end-stage disease. Thus, from the clinical point of view, PLV closure would not be indicated in critically-ill patients, those with acute endocarditis or, in general, those with a life expectancy of<1 year.
The clinical success in published series varies greatly, between 40% and 100%. In the largest series, the clinical success of survivors was 72%,13 which is identical to that of our series. There was a significant relation between the degree of residual valvular regurgitation and functional class, suggesting that percutaneous PVL closure, when successful, can be a wholly valid alternative to valve replacement surgery, with clear clinical improvement in the long-term.
Study LimitationsThe main limitation is that this study is based on the experience of a single center with a small number of patients. In addition, femoral access was used in all patients, given that transapical access began to be used in the hospital at a later date. This may have influenced the procedural success rate—which might increase with the introduction of transapical access. However, the strength of this work lies in the absence of studies with mid- to long-term follow-up of the new AVP III device which, in this series was used in 86% of cases.
CONCLUSIONSPercutaneous closure of PVL with the AVP III device offers significant long-term clinical improvement in a high percentage of patients. However, mortality was high in our population of high-risk patients, independently of procedure success, showing that PVL closure is indicated at a very advanced stage of the cardiovascular disease.
CONFLICTS OF INTERESTNone declared.