Systemic thrombolysis (ST) in acute pulmonary embolism (PE) at intermediate-to-high risk (IHR) and high risk (HR) improves clinical and hemodynamic parameters, but raises the risk of bleeds and is consequently underused.1 In an attempt to lower this risk and to increase the use of reperfusion therapies, catheter-directed therapies (CDT) are now starting to be used in the context of multidisciplinary groups known as PE rapid-response teams (PERT).2 At present, CDT is recommended for patients with a contraindication for ST and HR-PE (IIa C) and can be considered in cases of hemodynamic deterioration in IHR patients.3 Prior published experience shows positive and promising results.2
An observational prospective registry was created with consecutive inpatients at a single hospital as part of a PERT program and based on a protocol validated in the hospital by the thromboembolic disease commission. The on-duty interventional cardiology team for stroke code also handles CDT and, therefore, care is continuously available (24/7). The intensivist is the physician who activates PERT after diagnosis and consensus with the Emergency and Cardiology Departments. All registry patients signed a consent form explaining that the therapy was an alternative treatment to standard of care with anticoagulant therapy. Our PERT establishes performance of emergency CDT in HR patients (always with a contraindication for ST) and urgent CDT (less than 12hours) in IHR patients.
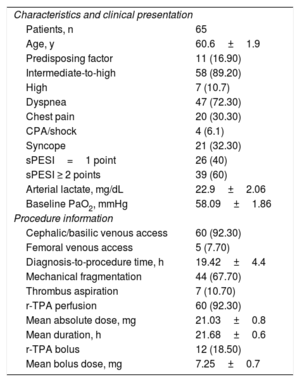
A total of 65 patients (table 1) were treated with CDT between 2017 and 2019 (90% of patients admitted to the ICU due to PE). Patients were included if they had IHR- or HR-PE confirmed by tomography according to the risk stratification criteria recommended by clinical practice guidelines, which include hemodynamic stability, elevated markers, and right ventricular involvement according to echocardiography parameters.
Sample characteristics
| Characteristics and clinical presentation | |
| Patients, n | 65 |
| Age, y | 60.6±1.9 |
| Predisposing factor | 11 (16.90) |
| Intermediate-to-high | 58 (89.20) |
| High | 7 (10.7) |
| Dyspnea | 47 (72.30) |
| Chest pain | 20 (30.30) |
| CPA/shock | 4 (6.1) |
| Syncope | 21 (32.30) |
| sPESI=1 point | 26 (40) |
| sPESI ≥ 2 points | 39 (60) |
| Arterial lactate, mg/dL | 22.9±2.06 |
| Baseline PaO2, mmHg | 58.09±1.86 |
| Procedure information | |
| Cephalic/basilic venous access | 60 (92.30) |
| Femoral venous access | 5 (7.70) |
| Diagnosis-to-procedure time, h | 19.42±4.4 |
| Mechanical fragmentation | 44 (67.70) |
| Thrombus aspiration | 7 (10.70) |
| r-TPA perfusion | 60 (92.30) |
| Mean absolute dose, mg | 21.03±0.8 |
| Mean duration, h | 21.68±0.6 |
| r-TPA bolus | 12 (18.50) |
| Mean bolus dose, mg | 7.25±0.7 |
CPA, cardiopulmonary arrest; PaO2, arterial pressure of oxygen; r-TPA, tissue plasminogen activator; sPESI, simplified PESI score.
Unless otherwise specified, the values are expressed as No. (%) or mean±SD.
At the operator's discretion and based on the thrombotic burden, hemodynamic status, and right-side pressures, CDT consisted of mechanical fragmentation, manual/mechanical aspiration, and/or local thrombolysis with tissue plasminogen activator (r-TPA) as bolus (2.5-10mg) and/or continuous infusion of 0.5-1mg/h (12-24hours) plus anticoagulant therapy with weight-adjusted sodium heparin. Patients with IHR-PE underwent local thrombolysis and fragmentation (5-6 Fr pigtail catheter) in the case of substantial occlusion of main or lobar arteries, whereas patients with HR-PE underwent both procedures plus aspiration (coronary intervention catheters). The latter was also performed in some IHR cases due to high thrombotic burden. The patients were reassessed by hemodynamic testing and angiography at 24hours.
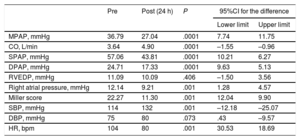
An efficacy endpoint was defined as a decrease of 5mmHg in mean pulmonary artery pressure and an increase of 0.5 L/min in cardiac output and was achieved in 90% of patients. Hemodynamic data before and after CDT were also analyzed, showing a 28% decrease in mean pulmonary artery pressure and a 34% increase in cardiac output. In addition, there was a decrease in thrombotic burden according to the Miller score (table 2). As a safety endpoint, the incidence of major bleed (BARC ≥ 3) was analyzed.
Hemodynamic outcomes (Student t test for comparison of paired samples)
| Pre | Post (24 h) | P | 95%CI for the difference | ||
|---|---|---|---|---|---|
| Lower limit | Upper limit | ||||
| MPAP, mmHg | 36.79 | 27.04 | .0001 | 7.74 | 11.75 |
| CO, L/min | 3.64 | 4.90 | .0001 | –1.55 | –0.96 |
| SPAP, mmHg | 57.06 | 43.81 | .0001 | 10.21 | 6.27 |
| DPAP, mmHg | 24.71 | 17.33 | .0001 | 9.63 | 5.13 |
| RVEDP, mmHg | 11.09 | 10.09 | .406 | –1.50 | 3.56 |
| Right atrial pressure, mmHg | 12.14 | 9.21 | .001 | 1.28 | 4.57 |
| Miller score | 22.27 | 11.30 | .001 | 12.04 | 9.90 |
| SBP, mmHg | 114 | 132 | .001 | –12.18 | –25.07 |
| DBP, mmHg | 75 | 80 | .073 | .43 | –9.57 |
| HR, bpm | 104 | 80 | .001 | 30.53 | 18.69 |
CO, cardiac output; DBP, diastolic blood pressure; DPAP, diastolic pulmonary artery pressure; HR, heart rate; MPAP, mean pulmonary artery pressure; RVEDP, right ventricle end-diastolic pressure; SBP, systolic blood pressure; SPAP, systolic pulmonary artery pressure.
There were 2 (3.07%) inpatient deaths: 1 due to a late (at 36 days) respiratory complication in a patient with Steinert disease and a respiratory muscle condition and 1 secondary to intracranial bleeding in a patient with syncope and traumatic brain injury. The 30-day mortality was 1.53% (only 1 inpatient death). The incidence of major hemorrhage was 3% (2 cerebral hemorrhages, both in patients with syncope and traumatic brain injuries).
Percutaneous treatment of PE is currently in the early stages, and there is no standard procedure or specific devices only for this indication. Patient profile as well as procedure timing and admissible delay are also unknown. However, the published evidence on CDT has reported consistent results for safety and efficacy.
Our results are similar to those of previous studies, with inpatient mortality similar to that seen in SEATTLE4 (3%) and below that reported in PERFECT5 (6%). Hemodynamic improvement is achieved with a low rate of bleeding complications, lower than that observed in ST trials. Studies such as SEATTLE4 or PERFECT5 have already shown that low-dose fibrinolytic and mechanical methods improved hemodynamics with a low bleeding risk.
The incidence of major bleeds with this approach is influenced by 3 factors: drug dosage and 24-hour distribution (compared with the usual r-TPA doses of 50-100μg within 1-2hours of ST), type of vascular access (only 7% by femoral vein vs 80% in SEATTLE4, potentially minimizing bleeds by making them readily identifiable and controllable), and type of clinical presentation. In our series, the 2 cerebral hemorrhages occurred after syncope with traumatic brain injury and, therefore, we believe that these patients should not be treated with CDT that includes thrombolysis.
In conclusion, despite the sample size limitations, the use of CDT in PE is safe and effective, and improves hemodynamic and clinical parameters, with an acceptable rate of bleeding complications. This registry may make way for larger registries that would contribute greater evidence.