The incidence of permanent pacemaker implantation (PPI) and new conduction abnormalities (CA) with the ACURATE neo (Symetis S.A., Eclubens, Switzerland) has not been studied in detail. We aimed to analyze their predictors, evaluating patient- and device-related factors, including implantation depth and device-to-annulus ratio (DAR).
MethodsTwo analyses of a multicenter population were performed: new PPI in pacemaker-naive patients (n = 283), and PPI/new-CA in patients without prior CA or pacemaker (n = 232).
ResultsA new PPI was required in 9.9% of patients, who had a higher body mass index, higher rate of right bundle branch block and bradycardia. Neither implantation depth nor DAR differed in patients with PPI compared with those without. In the multivariable analysis neither DAR (OR, 1.010; 95%CI, 0.967-1.055; P = .7) nor implantation depth (OR, 0.972; 95%CI, 0.743-1.272; P = .8) predicted PPI. Only high body mass index, bradycardia and right bundle branch block persisted as independent predictors. PPI/new-onset CA occurred in 22.8% of patients and was associated with a higher logistic EuroSCORE. Neither implantation depth nor DAR differed in patients with PPI/new-CA vs those without (7.3 ± 1.9 vs 7.1 ± 1.5mm; P = .6 and 41.0 ± 7.9 vs 42.2 ± 10.1%; P = .4). The only predictor of PPI/new-CA was a higher logistic EuroSCORE (OR, 1.039; 95%CI, [1.008-1.071]; P = .013).
ConclusionsNew PPI and new-onset CA rates were low with the ACURATE neo. These were mainly influenced by patient characteristics and not by device-depending factors.
Keywords
Cardiac conduction abnormalities (CA) leading to new permanent pacemaker implantation (PPI) are a frequent complication after transcatheter aortic valve implantation (TAVI).1 While earlier investigations as well as recent data from the SURTAVI trial have found no negative effect of new PPI on outcome,2,3 data from the PARTNER trial have identified chronic pacing as an independent predictor of 1-year mortality after TAVI.4,5 Moreover, PPI increases overall costs and is an important cause of prolonged hospital stay.4,6
The rate of new PPI with self-expanding transcatheter heart valves (THVs) has been thoroughly analyzed with older generation devices such as the CoreValve (Medtronic, Inc, Minneapolis, Minnesota, United States) with incidences of up to 40%.7 With the new generation of self-expanding THV, the PPI rate has decreased, showing rates of 12% to 15% for the CoreValve Evolut R (Medtronic, Inc, Minneapolis, Minnesota, United States) and 9% to 10% for the Portico (St. Jude Medical, St. Paul, Minnesota, United States).8–11
In 2014, a novel self-expanding THV, the ACURATE neo (Symetis S.A., Ecublens, Switzerland) obtained CE-mark, and postmarket registry data of 1000 patients showed a promising PPI rate of 8.2%.12 However, a detailed analysis of the PPI rate and possible underlying mechanisms has not been performed. Apart from nonmodifiable patient-related factors, such as prior right bundle branch block, atrioventricular block I or atrial fibrillation, which have been shown to influence PPI rates, device-specific mechanisms, such as implantation depth and the device-to-annulus ratio (DAR), may play an additional role.1
Therefore, we analyzed the association of a comprehensive set of clinical and electrocardiographic characteristics as well as multislice computed tomography-derived DAR and implantation depth with PPI and new-onset CA after TAVI with the ACURATE neo.
METHODSPatient Population and Definition of EndpointsBetween January 2014 and January 2016, 311 consecutive patients with severe native aortic valve stenosis underwent transfemoral TAVI with the ACURATE neo at 3 German centers.13 The endpoints of this study were: a) the need for PPI before discharge, and b) the composite of new PPI and/or new-onset CA (PPI/new-onset CA). For the new PPI analysis, patients with a prior pacemaker were excluded (n = 28) leaving 283 patients for analysis.
To analyze PPI/new-onset CA, patients with a prior pacemaker (n = 28), complete bundle branch block at baseline (n = 47), as well as incomplete electrocardiography data (n = 3) and procedural death (n = 1) were excluded, leaving 232 for analysis. New-onset CA was defined as new left bundle branch block or right bundle branch block before discharge.
A 12-lead electrocardiogram was performed on admission and before discharge and was reviewed by 2 physicians blinded to clinical data according to current recommendations.14 Doubtful cases were solved by consensus. In patients with a new PPI, intraventricular conduction was not evaluated due to potential interference of pacemaker stimulation and was denoted as “pacemaker”. Data were prospectively collected and classified according to the updated Valve Academic Research Consortium criteria (VARC-2).15
Multislice Computed Tomography AnalysisElectrocardiography-gated multislice computed tomography was performed in all cases either with the SOMATOM Force or SOMATOM Definition Flash (both Siemens Healthcare, Erlangen, Germany). Aortic annulus measurements were performed in multiple plane reconstruction according to current guidelines as previously described.16,17 In short, minimal and maximal diameters, annulus area and perimeter were determined at the nadir of the coronary cusps. Annulus eccentricity was assessed through the eccentricity index as [1 − minimum diameter/maximum diameter]. Calcification of the aortic cusps was visually graded and dichotomized as none/mild vs moderate/severe. Food and Drug Administration approved software OsiriX MD 3.9.4 (Pixmeo, Switzerland) or 3Mensio (3Mensio, Bilthoven, the Netherlands) were used.
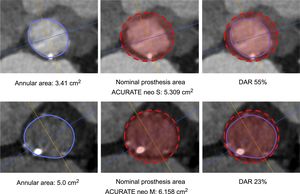
Prosthesis Size Selection and ProcedureThe ACURATE neo is available in 3 sizes, small, medium and large, covering an annulus range of 21mm to 27mm. The final decision of prosthesis selection was left at the discretion of the physician performing the procedure, taking into consideration the manufacturer's sizing recommendations, calcification, and anatomical features. Technical features and sizing recommendations are depicted in the Figure of the supplementary material. DAR was calculated as a surrogate for prosthesis oversizing using the formula: (nominal prosthesis dimension/patient's anatomy-1)*100.16 Adherence to the sizing guidelines according to area was categorized as “within range”, “undersized”, and “oversized”. Examples are given in Figure 1. The procedure was performed as previously described.18 All patients provided written informed consent for the procedure.
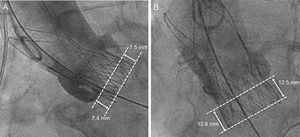
Prosthesis Depth AssessmentProsthesis implantation depth was assessed in a core laboratory (ISAResearch Center, Deutsches Herzzentrum München, Munich, Germany) using the final aortic angiogram showing the prosthesis in an orthogonal view as previously described.16 The native aortic annulus was marked by intersecting the nadir point of the sinuses of Valsalva. The prosthesis stent body height and the portion below the aortic annulus were measured at the septal (ie, noncoronary cusp) and nonseptal (ie, left coronary cusp) sides. Implantation depth was defined as the distance from the aortic annulus to the distal part of the prosthesis (Figure 2). QAngio XA Version 7.3 (Medis Medical Imaging Systems, Leiden, the Netherlands) with isocenter calibration was used for all measurements. Prosthesis depth was assessed for 276/283 (98%) patients with evaluable postdeployment aortic angiogram. When multiple valves were deployed (n = 6), the depth of the prosthesis deepest protruding into the left ventricular outflow tract was assessed.
Statistical AnalysisContinuous variables are expressed as mean ± standard deviation or as median [interquartile range] and were compared using the unpaired Student t test or Mann-Whitney U test as appropriate. Discrete variables were compared with the chi-square test or Fisher exact test as appropriate. To identify independent predictors for PPI and new-onset CA, multivariable analyses were performed, adjusted by variables yielding a P < .1 in univariate analyses. In order to assess the impact of DAR, implantation depth as well as atrioventricular block I, which have been described to influence PPI rates,1 these variables were included into the models independently of their P-value in univariate analyses. Due to multicollinearity between risk scores, EuroSCORE was the only risk score included in the multivariable analysis. Odds ratios (ORs) with 95% confidence intervals (95%CIs) were computed. A 2-sided P value of < .05 was considered statistically significant. IBM SPSS Statistics Version 22 (SPSS Inc, Chicago, Illinois, United States) was used for analyses.
RESULTSThe mean age of the whole study population was 80.8 ± 5.5 years, 61.1% (173/283) were female, and the mean logistic EuroSCORE and Society of Thoracic Surgeon Score were 17.0 ± 9.9% and 5.5 ± 4.1%, respectively. Mean implantation depth was 7.1 ± 1.6mm. Mean DAR was 42.2 ± 9.8% and prosthesis size selection was within range in 75.6%, undersized in 4.2%, and oversized in 20.2% of cases. Device success was achieved in 88.7% (251/283) and in-hospital mortality was 1.4% (4/283).
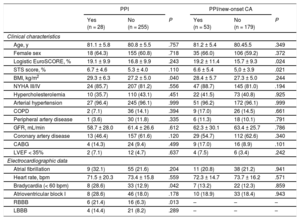
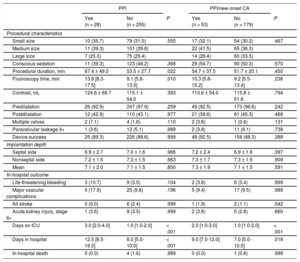
New Permanent Pacemaker ImplantationNew PPI was required in 9.9% (28/283) mostly due to persistent atrioventricular block III in 42.9% (12/28) and symptomatic bradycardia in 25.0% (7/28). For detailed PPI indication see Table 1 of the supplementary material. The PPI rates did not differ among participating centers (10.3%, 8.6%, and 9.9%; P for the trend .768). Patients with PPI had a higher body mass index (29.3 ± 6.3 vs 27.2 ± 5.0kg/m2; P = .040), a higher rate of bradycardia (heart rate < 60 bpm) on admission (28.6% vs 12.9%; P = .042) and of complete right bundle branch block (21.4% vs 6.3%; P = .013) compared with those without PPI (Table 1). Procedural duration (67.4 ± 49.0 vs 53.5 ± 27.7min; P = .022) and fluoroscopy time (13.8 [8.3-17.5] vs 9.1 [5.8-13.5] min; P = .010) were significantly longer in patients with new PPI compared with those without. There was no difference in use of conscious sedation or pre- and postdilatation strategy between the 2 groups. In-hospital outcome was similar in patients with PPI compared with those without (Table 2). Need for PPI was independent of prosthesis size selection (P for trend.555). Overall hospital stay was longer in patients requiring PPI (12.5 [8.5-16.0] vs 8 .0 [5.0-10.0] days; P < .001).
Baseline and Electrocardiography Characteristics
| PPI | PPI/new-onset CA | |||||
|---|---|---|---|---|---|---|
| Yes (n = 28) | No (n = 255) | P | Yes (n = 53) | No (n = 179) | P | |
| Clinical characteristics | ||||||
| Age, y | 81.1 ± 5.8 | 80.8 ± 5.5 | .757 | 81.2 ± 5.4 | 80.45.5 | .349 |
| Female sex | 18 (64.3) | 155 (60.8) | .718 | 35 (66.0) | 106 (59.2) | .372 |
| Logistic EuroSCORE, % | 19.1 ± 9.9 | 16.8 ± 9.9 | .243 | 19.2 ± 11.4 | 15.7 ± 9.3 | .024 |
| STS score, % | 6.7 ± 4.6 | 5.3 ± 4.0 | .110 | 6.6 ± 5.4 | 5,0 ± 3.9 | .021 |
| BMI, kg/m2 | 29.3 ± 6.3 | 27.2 ± 5.0 | .040 | 28.4 ± 5.7 | 27.3 ± 5.0 | .244 |
| NYHA III/IV | 24 (85.7) | 207 (81.2) | .556 | 47 (88.7) | 145 (81.0) | .194 |
| Hypercholesterolemia | 10 (35.7) | 110 (43.1) | .451 | 22 (41.5) | 73 (40.8) | .925 |
| Arterial hypertension | 27 (96.4) | 245 (96.1) | .999 | 51 (96.2) | 172 (96.1) | .999 |
| COPD | 2 (7.1) | 36 (14.1) | .394 | 9 (17.0) | 26 (14.5) | .661 |
| Peripheral artery disease | 1 (3.6) | 30 (11.8) | .335 | 6 (11.3) | 18 (10.1) | .791 |
| GFR, mL/min | 58.7 ± 28.0 | 61.4 ± 26.6 | .612 | 62.3 ± 30.1 | 63.4 ± 25.7 | .786 |
| Coronary artery disease | 13 (46.4) | 157 (61.6) | .120 | 29 (54.7) | 112 (62.6) | .340 |
| CABG | 4 (14.3) | 24 (9.4) | .499 | 9 (17.0) | 16 (8.9) | .101 |
| LVEF < 35% | 2 (7.1) | 12 (4.7) | .637 | 4 (7.5) | 6 (3.4) | .242 |
| Electrocardiographic data | ||||||
| Atrial fibrillation | 9 (32.1) | 55 (21.6) | .204 | 11 (20.8) | 38 (21.2) | .941 |
| Heart rate, bpm | 71.5 ± 20.3 | 73.4 ± 15.8 | .559 | 72.3 ± 14.7 | 73.7 ± 16.2 | .571 |
| Bradycardia (< 60 bpm) | 8 (28.6) | 33 (12.9) | .042 | 7 (13.2) | 22 (12.3) | .859 |
| Atrioventricular block I | 8 (28.6) | 46 (18.0) | .178 | 10 (18.9) | 33 (18.4) | .943 |
| RBBB | 6 (21.4) | 16 (6.3) | .013 | – | – | – |
| LBBB | 4 (14.4) | 21 (8.2) | .289 | – | – | – |
BMI, body mass index; CA, conduction abnormalities; CABG, coronary artery bypass graft; COPD, chronic obstructive pulmonary disease; GFR, glomerular filtration rate; LBBB, left bundle branch block; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; PPI, permanent pacemaker implantation; RBBB, right bundle branch block; STS, Society of Thoracic Surgeons.
Data are expressed as mean ± standard deviation or No. (%).
Procedural Characteristics and Outcome
| PPI | PPI/new-onset CA | |||||
|---|---|---|---|---|---|---|
| Yes (n = 28) | No (n = 255) | P | Yes (n = 53) | No (n = 179) | P | |
| Procedural characteristics | ||||||
| Small size | 10 (35.7) | 79 (31.0) | .555 | 17 (32.1) | 54 (30.2) | .467 |
| Medium size | 11 (39.3) | 101 (39.6) | 22 (41.5) | 65 (36.3) | ||
| Large size | 7 (25.0) | 75 (29.4) | 14 (26.4) | 60 (33.5) | ||
| Conscious sedation | 11 (39.3) | 123 (48.2) | .368 | 29 (54.7) | 90 (50.3) | .570 |
| Procedural duration, min | 67.4 ± 49.0 | 53.5 ± 27.7 | .022 | 54.7 ± 37.5 | 51.7 ± 20.1 | .450 |
| Fluoroscopy time, min | 13.8 [8.3-17.5] | 9.1 [5.8-13.5] | .010 | 10.3 [5.8-15.2] | 9.2 [5.5-13.4] | .236 |
| Contrast, mL | 124.6 ± 68.7 | 115.1 ± 54.0 | .393 | 113.6 ± 54.0 | 115.8 ± 51.6 | .794 |
| Predilatation | 26 (92.9) | 247 (97.9) | .259 | 49 (92.5) | 173 (96.6) | .242 |
| Postdilatation | 12 (42.9) | 110 (43.1) | .977 | 21 (39.6) | 81 (45.3) | .468 |
| Multiple valves | 2 (7.1) | 4 (1.6) | .110 | 2 (3.8) | 1 (0.6) | .131 |
| Paravalvular leakage II+ | 1 (3.6) | 13 (5.1) | .999 | 2 (3.8) | 11 (6.1) | .738 |
| Device success | 25 (89.3) | 226 (88.6) | .999 | 49 (92.5) | 158 (88.3) | .388 |
| Implantation depth | ||||||
| Septal side | 6.9 ± 2.7 | 7.0 ± 1.6 | .966 | 7.2 ± 2.4 | 6.9 ± 1.6 | .397 |
| Nonseptal side | 7.2 ± 1.5 | 7.3 ± 1.5 | .663 | 7.3 ± 1.7 | 7.3 ± 1.5 | .909 |
| Mean | 7.1 ± 2.0 | 7.1 ± 1.5 | .850 | 7.3 ± 1.9 | 7.1 ± 1.5 | .591 |
| In-hospital outcome | ||||||
| Life-threatening bleeding | 3 (10.7) | 9 (3.5) | .104 | 2 (3.8) | 6 (3.4) | .999 |
| Major vascular complications | 5 (17.9) | 25 (9.8) | .196 | 5 (9.4) | 17 (9.5) | .989 |
| All stroke | 0 (0.0) | 6 (2.4) | .999 | 1 (1.9) | 2 (1.1) | .542 |
| Acute kidney injury, stage II+ | 1 (3.6) | 9 (3.5) | .999 | 2 (3.8) | 5 (2.8) | .660 |
| Days on ICU | 3.0 [2.0-4.0] | 1.0 [1.0-2.0] | < .001 | 2.0 [1.0-3.0] | 1.0 [1.0-2.0] | < .001 |
| Days in hospital | 12.5 [8.5-16.0] | 8.0 [5.0-10.0] | < .001 | 9.0 [7.0-12.0] | 7.0 [5.0-10.0] | .016 |
| In-hospital death | 0 (0.0) | 4 (1.6) | .999 | 0 (0.0) | 1 (0.6) | .999 |
CA, conduction abnormalities; ICU, intensive care unit; PPI, permanent pacemaker implantation.
Data are expressed as No. (%), mean ± standard deviation, or median [interquartile range].
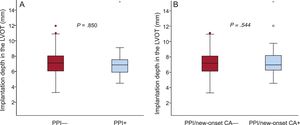
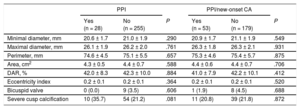
Angiographic core laboratory analysis revealed that mean implantation depth did not differ in patients with PPI compared with those without (7.1 ± 2.0 vs 7.1 ± 1.5mm; P = .850) (Table 2 and Figure 3A). Multislice computed tomography data and the degree of DAR according to need for PPI are displayed in Table 3. There was a nonsignificant trend of higher rates of severe cusp calcification in patients with need for PPI than in those without (35.7% vs 21.2%; P = .081), whereas there was no difference in aortic anatomy in terms of bicuspid valves and annular eccentricity.
Multislice Computed Tomography Measurements of the Aortic Annulus and Oversizing
| PPI | PPI/new-onset CA | |||||
|---|---|---|---|---|---|---|
| Yes (n = 28) | No (n = 255) | P | Yes (n = 53) | No (n = 179) | P | |
| Minimal diameter, mm | 20.6 ± 1.7 | 21.0 ± 1.9 | .290 | 20.9 ± 1.7 | 21.1 ± 1.9 | .549 |
| Maximal diameter, mm | 26.1 ± 1.9 | 26.2 ± 2.0 | .761 | 26.3 ± 1.8 | 26.3 ± 2.1 | .931 |
| Perimeter, mm | 74.6 ± 4.5 | 75.1 ± 5.5 | .657 | 75.3 ± 4.6 | 75.4 ± 5.7 | .875 |
| Area, cm2 | 4.3 ± 0.5 | 4.4 ± 0.7 | .588 | 4.4 ± 0.6 | 4.4 ± 0.7 | .706 |
| DAR, % | 42.0 ± 8.3 | 42.3 ± 10.0 | .884 | 41.0 ± 7.9 | 42.2 ± 10.1 | .412 |
| Eccentricity index | 0.2 ± 0.1 | 0.2 ± 0.1 | .364 | 0.2 ± 0.1 | 0.2 ± 0.1 | .520 |
| Bicuspid valve | 0 (0.0) | 9 (3.5) | .606 | 1 (1.9) | 8 (4.5) | .688 |
| Severe cusp calcification | 10 (35.7) | 54 (21.2) | .081 | 11 (20.8) | 39 (21.8) | .872 |
CA, conduction abnormalities; DAR, device-to-annulus ratio; PPI, permanent pacemaker implantation.
Data are expressed as No. (%) or mean ± standard deviation.
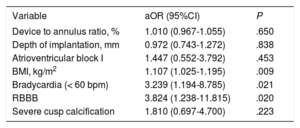
In the multivariable analysis, only body mass index, bradycardia and complete right bundle branch block at baseline persisted as independent predictors of PPI (Table 4). Device-to-annulus ratio (OR, 1.010; 95%CI, 0.967-1.055; P = .650), implantation depth (OR, 0.972; 95%CI, 0.743-1.272; P = .838) and atrioventricular block I (OR, 1.447; 95%CI, 0.552-3.792; P = .453) were not associated with new PPI. Furthermore, the PPI rate was constant across the tertiles of consecutive procedures, indicating no effect of a learning curve on PPI rates (P for trend .845).
Multivariate Analysis for New Permanent Pacemaker Implantation
| Variable | aOR (95%CI) | P |
|---|---|---|
| Device to annulus ratio, % | 1.010 (0.967-1.055) | .650 |
| Depth of implantation, mm | 0.972 (0.743-1.272) | .838 |
| Atrioventricular block I | 1.447 (0.552-3.792) | .453 |
| BMI, kg/m2 | 1.107 (1.025-1.195) | .009 |
| Bradycardia (< 60 bpm) | 3.239 (1.194-8.785) | .021 |
| RBBB | 3.824 (1.238-11.815) | .020 |
| Severe cusp calcification | 1.810 (0.697-4.700) | .223 |
95%CI, 95% confidence interval; aOR, adjusted odds ratio; BMI, body mass index; bpm, beats per minute; RBBB, right bundle branch block.
After discharge only 1 patient required PPI at 30 days follow-up due to sick sinus syndrome, leading to a cumulative PPI rate of 10.2% (29/283) at 30 days.
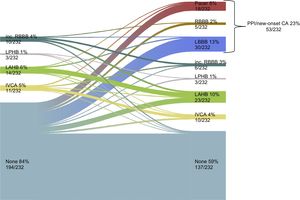
New Permanent Pacemaker Implantation or New-onset Conduction AbnormalitiesPermanent pacemaker implantation/new-onset CA occurred in 22.8% (53/232) of cases. Changes in cardiac conduction before and after TAVI is depicted in Figure 4. Patients with new PPI/new-onset CA had a higher logistic EuroSCORE than patients without (19.2 ± 11.4 vs 15.7 ± 9.3%; P = .024) (Table 1). Procedural characteristics and outcome according to PPI/new-onset CA are depicted in Table 2. Selected prosthesis size (P for trend .467) did not differ in patients with PPI/new-onset CA, whereas hospital stay was significantly longer (9.0 [7.0-12.0] vs 7.0 [5.0-10.0] days; P = .016). Pre- and postdilatation did not differ in the 2 groups (Table 2). Between the 2 groups neither implantation depth (mean implantation depth 7.3 ± 1.9 vs 7.1 ± 1.5mm; P = .591) (Table 2 and Figure 3B) nor DAR (41.0 ± 7.9 vs 42.2 ± 10.1%; P = .412) (Table 3) differed.
Evolution of cardiac conduction at baseline and before discharge in the population for PPI/new-onset CA analysis. CA, conduction abnormalities; IVCA, intraventricular conduction abnormality; LAHB, left anterior hemiblock; LBBB, left bundle branch block; LPHB, left posterior hemiblock; PPI, permanent pacemaker implantation; RBBB, right bundle branch block.
In a multivariable analysis, only logistic EuroSCORE (OR, 1.039; 95%CI, 1.008-1.071; P = .013) persisted as an independent predictor of PPI/new-onset CA, whereas neither DAR (OR, 0.988; 95%CI, 0.954-1.023; P = .502), nor implantation depth (OR, 1.068; 95%CI, 0.875-1.303; P = .520) or atrioventricular block I (OR, 1.008; 95%CI, 1.008-1.071; P = .986) predicted PPI/new-onset CA (Table 5). New-onset left bundle branch block occurred in 12.9% (30/232) of cases. A multivariable model was computed to assess risk predictors for isolated new-onset left bundle branch block (Table 2 of the supplementary material) and a higher logistic EuroSCORE was the only predictor (OR, 1.038; 95%CI, 1.002-1.076; P = .038). The rate of PPI/new-onset CA was stable across the tertiles of consecutive procedures, indicating no effect of a learning curve (P for trend .237).
Multivariate Analysis for Permanent Pacemaker Implantation/new-onset Conduction Abnormalities
| Variable | aOR 95%CI | P |
|---|---|---|
| Device-to-annulus ratio, % | 0.988 (0.954-1.023) | .502 |
| Depth of implantation, mm | 1.068 (0.875-1.303) | .520 |
| Atrioventricular block I | 1.008 (1.008-1.071) | .986 |
| Logistic EuroSCORE % (per each % increase) | 1.039 (1.008-1.071) | .013 |
95%CI, 95% confidence interval; aOR, adjusted odds ratio.
For the first time, we analyzed the incidence and predictors of PPI and new-onset CA with the ACURATE neo THV in a multicenter population, focusing particularly on the influence of DAR and implantation depth. Our findings show low rates of both endpoints. Angiographic core laboratory and multislice computed tomography data analysis revealed no influence of implantation depth or DAR on PPI and new-onset CA, which appears to be primarily determined by patient-related factors, especially by baseline electrocardiography variables (complete right bundle branch block and baseline bradycardia).
Permanent Pacemaker ImplantationsWhile some investigations showed no effect of PPI on mortality,3 a recent analysis of the PARTNER trial identified chronic pacing as an independent predictor of 1-year mortality after TAVI.4,5 Therefore, a reduction in PPI rates is paramount, especially when extending indications toward a younger, lower risk population. With the ACURATE neo, we report a rate of PPI of 9.9%, consistent with registry data of 8.2%.12 More recently, a very low PPI rate of 2.3% using the ACURATE neo has been described.19 Although this analysis comprised only 175 patients, it suggests that an even lower PPI rate can be achieved. It needs to be considered that our analysis features the initial experience with this THV, while the lower PPI rate comprises patients treated more recently. Permanent pacemaker implantation rates change over time with increasing operator experience. For example, the initial PPI rate with the SAPIEN 3 ranged from 13% to 21%,20,21 whereas more recent experience reported rates as low as 9.9% and 13.2% at 1 year.22,23 Further research will assess whether increasing operator experience and a different implantation technique result in lower PPI rates with the ACURATE neo.
Studies on other next-generation self-expanding THVs have reported PPI rates of 12% to 15% for the Evolut R8,9 and 9% to 10% for the Portico.10,11 To date no data are available from randomized trials directly comparing next-generation THV regarding PPI rates. Several clinical trials addressing this issue are ongoing, namely, the SCOPE I (Safety and Efficacy of the Symetis ACURATE Neo/TF Compared to the Edwards SAPIEN 3 Bioprosthesis) registered at ClinicalTrials.gov (Identifier: NCT03011346); the SCOPE II (Safety and Efficacy Comparison Of Two TAVI Systems in a Prospective Randomized Evaluation II) registered at ClinicalTrials.gov (Identifier: NCT03192813), and the SOLVE-TAVI (SecOnd-generation seLf-expandable Versus Balloon-expandable Valves and gEneral Versus Local Anesthesia in TAVI) registered at ClinicalTrials.gov (Identifier: NCT02737150) and will provide further insights on this topic. Furthermore, these randomized comparisons will allow an extensive comparison of THVs beyond the PPI rate, evaluating clinical outcome and device success. In this analysis, we acknowledge a VARC-2 defined device success of 89%, which at first glance may appear low compared with other reported rates from large studies ie, the PARTNER trials. However, many studies do not report the VARC-2 defined device success, making interstudy comparison difficult. Studies that do report this endpoint showed similar rates of device success for the ACURATE neo (89.1%), the SAPIEN 3 (75.7%-90.4%) and for the LOTUS Valve (77.1%).13,24 An important contributor to device success is paravalvular leakage, which in this analysis was 4.9%. Currently, a next-generation THV, the ACURATE neo AS, is enrolling patients to achieve CE-mark. This device is featured with an additional sealing skirt to reduce paravalvular leakage. Future studies will elucidate whether a lower rate of paravalvular leakage and hence higher device success can be achieved, without leading to a higher PPI rate.
Multiple predictors for PPI after TAVI have been described in the literature. In a recent meta-analysis of PPI, Siontis et al. categorized these into patient-related, electrocardiographic, and procedural factors. While the former 2 categories cannot be influenced by the operator's choices or skills, procedural or device-related factors may be influenced by sizing or implantation technique.1
The influence of implantation depth on PPI has been described for different THV. In the case of CoreValve Evolut R, patients requiring PPI had a mean implantation depth of 9mm at the noncoronary cusp,8 while an implantation depth < 7mm has been associated with lower PPI rates for the CoreValve.7 Considering balloon-expandable valves, such as the SAPIEN 3, a cutoff of 8mm has been revealed to predict the need for PPI.20 In the present study, we acknowledge a protrusion into the left ventricular outflow tract with a mean depth of 7mm; notably, there was no association of implantation depth with new PPI or PPI/new-onset CA.
The ACURATE neo is deployed in 2 steps with a top-down release, first opening 3 stabilization arches in the ascending aorta and the upper crown, then in a second step, the lower part of the prosthesis is released in the left ventricular outflow tract. This top-down release, which stands in contrast to most currently used self-expanding THV, may result in less mechanical trauma to the conduction system.
To date, the influence of DAR or prosthesis oversizing has been evaluated primarily in the context of paravalvular leakage. As a self-expanding system, the ACURATE neo anchors in the aortic annulus by exerting a continuous radial force on the surrounding valvular apparatus and certain degree of oversizing is required to avoid paravalvular leakage. However, its influence on new PPI is still unclear. Experience with the CoreValve prosthesis showed no influence of DAR on PPI rates.25 In the present analysis, DAR was relatively high–up to 40% by area–but no effect was observed on PPI rates. This finding may not be surprising, if we consider that self-expanding THVs exert a lower radial force and adapt to the patient's anatomy, exerting less pressure on the surrounding tissue and causing less damage to the conduction system. A further possible explanation is that a larger DAR does not negatively affect the conduction system as the prosthesis is implanted within the aortic annulus and exerts a low radial force especially on the ventricular extremity of the THV.
The present study shows that the risk of PPI is mainly influenced by patient-related factors. These include a high body mass index, baseline bradycardia, and pre-existing complete right bundle branch block. In particular, prior complete right bundle branch block has been consistently reported as a strong predictor of PPI, regardless of the THV model used1. Presumably device induced traumatic injury to a degenerated conduction system (visible as right bundle branch block or bradycardia) leads to complete heart block with requirement of new PPI. The observation of a high body mass index influencing PPI rate may be a chance finding requiring confirmation in other studies. However, one explanation may be that adipose patients are generally at higher risk for cardiovascular diseases as well as CA.26
Conduction disturbances after TAVI are dynamic and a proportion of patients are at higher risk for late PPI, while in some patients, CA may resolve not requiring PPI at all.27 We found a stable rate of PPI of 10.2% at 30 days. This may be explained by the fact that this THV exerts a lower radial force compared with other self-expanding THVs and therefore does not apply prolonged stress on the underlying conduction system.
New-onset Conduction AbnormalitiesNew-onset or worsened CA have been described as a common complication following TAVI. The underlying mechanisms include direct injury to the conduction system, but also the intrinsic degeneration and calcification of the conduction system, which are highly prevalent in the elderly TAVI population. This may also explain the finding that a higher logistic EuroSCORE, reflecting an older and sicker population, predicts PPI/new-onset CA.
Most of the analyses conducted on new CA after TAVI have focused on new complete bundle branch blocks, especially new-onset left bundle branch block. This may be because the presence of new left bundle branch block after TAVI negatively affects long-term survival with an increased rate of cardiac death, left ventricular dysfunction, and increased need for PPI at 1 year.5,28 Therefore, it is of importance to minimize CA to prolong event-free survival.
The incidence of new-onset left bundle branch block has been reported in a range from 8% to 30% with balloon-expandable valves and is even higher with self-expanding devices such as the CoreValve from 22.2% to up to 50.0%.16,29–31 In this analysis, we report a PPI/new-onset CA rate of 22.8%. In particular, new-onset left bundle branch block occurred in 12.9% of patients, which is lower than the reported incidence for other self-expanding THV. This could be explained by higher implantation as well as lower radial force of the ACURATE neo and therefore less mechanical trauma to the conduction system. Considering new-onset left bundle branch block, only a higher logistic EuroSCORE was an independent predictor in the multivariate analysis, whereas other previously described predictors such as coronary artery bypass graft did not influence left bundle branch block rates.30
The incidence of new-onset CA after TAVI beyond complete left bundle branch block has been reported in several studies,16,31 but its influence on outcome has not yet been thoroughly analyzed. Moving toward a younger population, future studies are necessary to assess whether these “minor” CA affect long-term outcome.
LimitationsA limitation of this analysis is the small number of PPI and new-onset CA, which reduce the statistical ability to identify risk predictors for these endpoints. However, this is the first report to specifically address this question using this novel THV. Larger trials are warranted to further address this issue. Furthermore, although core laboratory assessment of fluoroscopic implantation depth was performed, this measurement is not always well predictable and may be hard to quantify.32 New-onset CA after TAVI may resolve over time,27 in this analysis we focused on the discharge electrocardiogram, and therefore transient CA were not considered.
CONCLUSIONSThis is the first analysis of predictors of PPI and new-onset CA with the ACURATE neo THV and shows low rates for both endpoints. In a comprehensive analysis, we found that the need for PPI and new-onset CA seems to be mainly affected by patient-related characteristics and not by operator or device-related factors such as prosthesis oversizing or implantation depth.
CONFLICTS OF INTERESTC. Pellegrini declares minor travel grants from Symetis S.A.; O. Husser declares minor travel grants, proctor and minor lecturing fees from Symetis S.A.; W.-K. Kim declares proctor fees from Symetis S.A. and St. Jude Medical and minor lecturing fees from Edwards Lifesciences; T. Trenkwalder declares minor travel grants from Symetis S.A.; C. Burgdorf declares proctor fees from Symetis S.A.; M. Hilker declares proctor fees from Symetis S.A.; H. Möllmann declares proctor fees and speaker honoraria from Symetis S.A. and C. Hengstenberg declares proctor fees and speaker honoraria from Symetis S.A.
- -
Cardiac CA leading to new PPI are a frequent and important complication after TAVI. Using the novel self-expanding ACURATE neo, postmarket registry data of 1000 patients showed a promising PPI rate of 8.2%. However, a detailed analysis of the PPI rate and of possible underlying mechanisms has not been performed.
- -
This is the first analysis of the incidence and predictors of PPI and new-onset CA with the ACURATE neo THV and shows low rates for both endpoints. In a comprehensive analysis, we found that need for PPI and new-onset CA seems to be mainly affected by patient-related characteristics and not by operator or device-related factors such as prosthesis oversizing or implantation depth.