Keywords
INTRODUCTION
Studies have shown the association between patent foramen ovale (PFO) and cryptogenic stroke. Currently, treatment of these patients is controversial, although data exist that favor percutaneous PFO closure.1 The objective of the present report is to study immediate and long-term follow-up results for a consecutive series of patients with cryptogenic stroke and percutaneous PFO closure.
METHODS
From January 2000 thru February 2006, 52 patients aged <60 years diagnosed with PFO and stroke underwent percutaneous closure in our hospital. All were diagnosed with acute stroke or transient ischemic attacks (TIA), and underwent full clinical examination (Table 1). Diagnosis of cardioembolic pathology was ruled out by echography. Foramen permeability was analyzed with Gelafundin 4% injected via a peripheral vein in baseline conditions and following the Valsalva maneuver.
Percutaneous foramen closure was indicated in patients with cryptogenic stroke, PFO, some of the following echocardiographic criteria, and considered at risk of new neurologic event as described elsewhere2,3: recurrence of neurologic clinical events, substantial contrast shunting at rest (>20 microbubbles), interatrial septal aneurysm (ISA) defined as 311 mm total excursion in left and/or right atrium,4 and substantial septum primum membrane mobility (>6 mm).
Implantation was undertaken with angiographic control and general anesthesia due to the difficulty entailed in keeping patients under transesophageal echocardiographic (TEE) monitoring during the procedure. Prior to implantation, pulmonary artery angiography in cava inferior was conducted to evaluate spontaneous left-to-right and right-to-left shunting (Figure 1). Later echographic bubble contrast was injected to determine right-to-left shunting by TEE in baseline conditions and after the forced expiratory respiration maneuver; this was repeated after implantation (Figure 1).
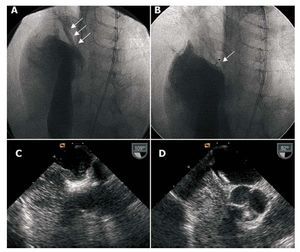
Figure 1. A: baseline angiography with contrast injection in right atrium showing right-to-left shunting. B: angiography of implanted device, sealing foramen ovale, with contrast injection in right atrium. C: transesophageal echography of right-to-left contrast shunting. D: transesophageal echography of implanted device, without right-to-left shunting.
We chose to deploy devices with a major diameter smaller than the total interatrial septum length determined by TEE retroaortic projection (Figure 2). In patients with septal aneurysm, we opted for a device slightly larger than the aneurysm base measure. Choice of device to deploy— Amplatzer (AGA Medical, Minnesota, USA), Intrasept (Cardia Inc., Minnesota, USA), or Helex (W.L. Gore, Arizona, USA)—was left to individual operators except in patients with septal aneurysm, when Amplatzer devices were always used. We had access to 18, 25, and 35 mm Amplatzer devices and 20, 25, and 30 mm Helex devices.
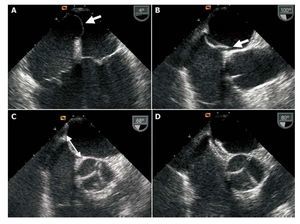
Figure 2. Transesophageal echography. A: aneurysm of interatrial septum. B: anatomic patent foramen ovale (arrow). C: measuring septum to choose device size (arrow). D: image of implanted device.
All patients received prophylactic antibiotic treatment following implantation and antithrombotic treatment with dalteparin (10 000 IU/day for 1 month) and aspirin (150 mg/day indefinitely).5,6
We conducted transthoracic echography (TTE) using second harmonic imaging and contrast prior to discharge and clinical follow-up and contrast echography at 6 months and 1 year, and in subsequent annual clinical follow-ups.
We present quantitative variables as mean (SD) and qualitative variables as percentages of the total. Data analysis was with SPSS 8.0.
RESULTS
We performed percutaneous PFO closure in 52 patients aged <60 years. Table 2 presents their clinical and echographic characteristics. Devices were successfully implanted in all patients. We used Amplatzer devices in 40 (76%) patients, Helex in 11 (22%), and Intrasept in 1. In 4 patients, we used an Amplatzer device for cribriform interatrial communication closure as they presented multiple membrane perforations. Device size was 35 mm in 25% of patients, 30 mm in 12%, 25 mm in 54%, and £20 mm in 10%.
All patients with ISA presented a base measure of >25 mm, so we used 35 mm Amplatzer devices. At the end of the procedure, we found residual bubble shunting in 12 patients (24%) in baseline situation and in 25 patients (48%) following forced expiration; this was considered mild in 11 patients.
No major complications or device embolization occurred. At discharge, we found residual shunting in 2 (4%) patients using contrast TTE.
We have conducted follow-up of 49 patients for a median of 26 months (range, 2-72; percentiles 25-75, 8-39). Three patients were lost to follow-up.
No clinical neurologic events, or events of any other type attributable to the procedure or device (endocarditis, embolization, fracture) occurred. At 6 months, the late TTE study found no residual shunting in any patient.
DISCUSSION
Since Overell et al's meta-analysis,7 the association between PFO and cryptogenic stroke has been clear and contrast TEE has proven the most adequate method of diagnosis.8 In patients with PFO, several studies have described risk factors for stroke3 similar to those we used as indications for closure.9
In the absence of randomized studies comparing medical treatment with percutaneous closure, the high rate of recurrence with medical treatment, as much as 13%,10 and the existence of studies that favor percutaneous closure11 make this a widely-used option. In Spain, in 2005, 182 percutaneous PFO closure procedures were performed.12
Incidence of recurrence following closure varies, ranging from 0.6% to 7.8%.11 In our study, there has been no recurrence. This may be due to the antithrombotic drug regimen used—not described in other studies— possible favoring adequate, rapid sealing of the device without thrombotic aggregation. Despite residual shunting following discharge in 4%, at 6-month echocardiographic follow-up no patient presented shunting, which coincides with results described elsewhere.13 In contrast, the major complications associated with percutaneous closure— in 1.5% of patients in the series described1—were not present in our study.
While procedures were conducted with TEE guidance, anesthesia can be avoided by using intracavity echography.
In our experience, percutaneous closure is a safe option in young patients with cryptogenic stroke and PFO, without recurrence or events in the long-term.
Limitations
The absence of a control group of patients with cryptogenic stroke and PFO treated medically prevents us from comparing both therapeutic options, but the rate of recurrence of neurologic events in patients with medical treatment was what motivated our decision to use percutaneous closure. The late evolution of these patients seems to endorse this decision. The finalization of randomized studies under way at the time of writing, and prolonged follow-up should lead to a more definitive ratification of these findings.
Correspondence:
Dr. F. Mazuelos Bellido.
Servicio de Cardiología. Hospital Reina Sofía. Avda. Menéndez Pidal, s/n. 14004 Córdoba. España.
E-mail: franciscomazuelos@gmail.com
Received September 12, 2007.
Accepted for publication November 7, 2007.