We report our experience in the surgical correction of anomalous origin of left coronary artery from pulmonary artery (ALCAPA), with an emphasis on the coronary reimplantation technique and its outcome.
MethodsWe designed a retrospective, longitudinal, descriptive study that included patients with ALCAPA who underwent surgery involving coronary reimplantation over a 19-year period. We describe perioperative details such as variations in the surgical technique and the postoperative outcome in terms of morbidity and mortality.
ResultsWe studied 15 patients (86% females) with a mean age of 6.2 years (range, 2 months to 24 years). Heart failure was the principal cause for hospitalization in 80% of our patients. Left ventricular dysfunction was present in 67%, and 27% had significant or severe mitral valve regurgitation. We describe 3 surgical techniques for coronary reimplantation, the choice of which depends on the site of origin of the anomalous left coronary artery. Four patients underwent an additional mitral valve procedure. The most common immediate postoperative complications were low cardiac output (38%), pleural effusion (17%), and transient ischemia (13%). There was no operative or medium-term mortality.
ConclusionsCoronary reimplantation is the technique of choice for surgical correction of ALCAPA due to the excellent postoperative survival and low operative morbidity.
Keywords
.
INTRODUCTIONCoronary artery anomalies constitute 2.2% of the cases of congenital heart disease, and the defect that occurs with the greatest frequency is anomalous origin of the coronary artery from the pulmonary artery (ALCAPA). The incidence of this entity ranges from 1 in 30 000 to 1 in 300 000 newborn infants,1 the great majority of whom die of severe heart failure during the first year of life if they do not receive proper treatment.2, 3, 4, 5
Surgery is the treatment of choice during the neonatal period or infancy because of the good results, which are capable of modifying the natural progression of the disease.6, 7, 8 A number of surgical techniques have been developed, but the most useful are those designed to restore the cardiac circulation in both coronary arteries. The technique most widely employed is that involving coronary reimplantation,5 which has been adopted by many centers as the technique of choice.7
The objective of this study is to report our experience in the surgical treatment of ALCAPA with coronary reimplantation, stressing the surgical technique and the postoperative outcomes obtained in terms of morbidity and mortality.
METHODS Study Design and Preoperative EvaluationA retrospective, longitudinal, descriptive study was carried out in the patients who underwent coronary reimplantation to correct ALCAPA from January 1987 to June 2010. Patients treated in centers outside the Mexican Social Security Institute (Instituto Mexicano del Seguro Social [IMSS]) and those in whom a technique other than coronary reimplantation was employed were excluded. The preoperative evaluation included demographic data, time elapsed since the onset of the symptoms and diagnosis on admission, functional class according to the New York Heart Association (NYHA) (for elementary school children and adolescents),9 and the modification suggested by Ross et al.10 (for newborns, infants and preschool children). The degree of heart failure was classified as decompensated (when intravenous inotropic agents and/or mechanical ventilation were used during the preoperative period, with a functional class of III or IV), compensated (when the patient was in functional class I or II, with oral diuretics, digitalis and/or vasodilators), or absent (if the patient did not require medication and was in functional class I).
The diagnostic techniques employed were electrocardiography, chest X-ray, echocardiography, and cardiac catheterization. None of the patients underwent spiral computed tomographic angiography. The electrocardiogram was studied for signs of ischemia or infarction and their location. In the chest X-ray, we looked for evidence of cardiomegaly and signs of congestion and/or acute pulmonary edema. Cardiomegaly was classified according to the cardiothoracic ratio as mild (≥0.55 and <0.65), moderate (≥0.65 and <0.75), or severe (≥0.75). The echocardiogram was examined to evaluate ventricular function and the presence and severity of mitral regurgitation. Mitral regurgitation was graded using a semiquantitative method based on the length and maximum width of the regurgitant jet with respect to left atrium as follows: 0 (no regurgitation), grade 1 (mild), grade 2 (moderate), grade 3 (significant), and grade 4 (severe).11 The degree of ventricular dysfunction was graded by measuring the left ventricular ejection fraction (LVEF) using Simpson's method, considering the normal range to be 65% to 70% and values of ≥45% to <65% to indicate mild dysfunction; 30% to <45%, moderate dysfunction; and <30%, severe dysfunction.12 A hemodynamic study was carried out to define the position of the anomalous left coronary artery and to measure the left ventricular end-diastolic pressure (LVEDP), taking 14mmHg as the maximum normal value.
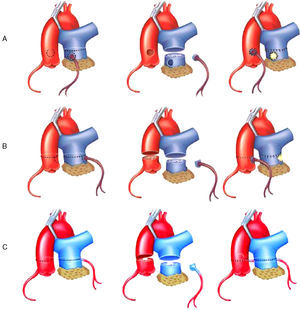
Surgical TechniqueThe surgical approach involved median sternotomy. The cardiopulmonary bypass circuit was established with aortic and bicaval cannulation. Following aortic clamping, myocardial protection was provided with hypothermia and administration of a cardioplegic solution. Once cardioplegic arrest had been achieved, we proceeded to determine the site of origin of the anomalous left coronary artery and 3 variants were distinguished: inner, lateral, and posterior. The strategy for coronary reimplantation for each of these variations was different. All of them involved transection of the pulmonary trunk (PT) above the origin of the anomalous left coronary artery. When the origin was in the inner aspect of the PT wall (Figure 1A), the coronary button was resected using a circular incision and the defect in the PT wall was closed with a bovine pericardial patch. The coronary button was mobilized in search of the most appropriate site in the anterior aortic wall; with care to prevent it from kinking, a 4 or 5-mm hole was punched in the aortic wall and the coronary button was reimplanted using a continuous nonabsorbable monofilament suture. When the coronary anomaly originated in the lateral wall (Figure 1B), making its mobilization more difficult, both the pulmonary and aortic trunks were transected. The coronary button was resected from the PT using an inverted D-shaped incision similar to that employed for the arterial switch operation. After closure of the PT defect with a pericardial patch, end-to-end anastomosis of the two transected arterial trunks was carried out. Lastly, in the variant originating in posterior wall (Figure 1C), pulmonary artery transection was performed to resect the coronary button as in the second variant, but the procedure differed in that a transverse incision was made in aortic trunk that involved only two thirds of the circumference of the vessel. A hole was punched in the posterior segment at the site most suitable for the reimplantation. Finally, aortic repair and end-to-end pulmonary artery anastomosis were completed. When grade 3 or 4 mitral regurgitation was present, annuloplasty was performed. In cases of mitral annuloplasty failure or unfavorable mitral valve anatomy, a prosthetic valve was placed. Following the mitral valve procedure, intraoperative transesophageal echocardiography (ITEE) was carried out to assess the function of left ventricle and of the implanted prosthesis or the repaired mitral valve.
Figure 1. Surgical technique for the correction of anomalous origin of left coronary artery from pulmonary artery trunk by coronary reimplantation, depending on the site of origin of the anomalous coronary artery. A: inner aspect of pulmonary trunk wall. B: lateral wall. C: posterior wall.
Immediate Postoperative Follow-up Strategies and Statistical AnalysisOperative mortality was considered to encompass all those patients who died within 30 operative morbid events were defined in accordance with the international nomenclature.13 The durations of the entire hospital stay, intensive care unit stay, use of mechanical ventilation, and postoperative hospital stay were also recorded.
The information obtained from the medical records was processed using an Excel spreadsheet. The data were presented as the mean, range of variation, and percentage of the population at risk. Parents or guardians of pediatric patients provided informed consent for the performance of the operation. The study of this clinical series was carried out with the authorization of the ethics committee of our institution.
RESULTS Results of The Preoperative EvaluationFifteen patients were included in this study, 13 females (86%) and 2 males (14%), a ratio of 6:1. The mean age was 6.2 years (2 months to 24 years), with a mode of 1.7 years. The mean body weight was 19.4kg (range: 3.6 to 47kg); the mean height, 98.8cm (52 to 176cm), and the mean body surface, 0.69 m2 (0.22 to 1.8 m2). Eighty percent of our patients with ALCAPA (12 cases) were under 12 years of age.
The age at the onset of symptoms was 14 months (1 to 17 months), but the majority of the patients (70%) developed symptoms prior to the age of 8 months. At hospital admission, 20% (3 cases) did not have heart failure, 67% (10 cases) had compensated heart failure, and only 2 (13%) had decompensated heart failure; these 2 patients received dobutamine on the 2 days prior to surgery and 1 of them required preoperative mechanical ventilation for 48h.
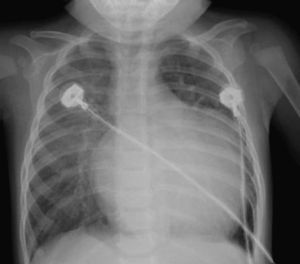
Table 1 shows the characteristics of the preoperative tests. The chest X-ray revealed cardiomegaly due to dilation of the left chambers in 12 cases (Figure 2), and only 2 patients (13%) had severe cardiomegaly with associated pulmonary congestion; in no case was acute pulmonary edema observed.
Table 1. Preoperative Tests in Patients With Anomalous Origin of Left Coronary Artery From Pulmonary Artery.
| Preoperative test | Results | Patients, no (%) |
| Electrocardiogram | No signs of ischemic lesion or infarction | 4 (27) |
| Anterior wall ischemia | 2 (13) | |
| Left lateral wall and posterior wall ischemia | 7 (47) | |
| Left lateral and posterior infarction | 2 (13) | |
| Chest X-ray | No cardiomegaly (CTR, 0.5-0.55) | 3 (20) |
| Mild cardiomegaly (CTR, ≥0.55-<0.65) | 4 (27) | |
| Moderate cardiomegaly (CTR, ≥0.65-<0.75) | 6 (40) | |
| Severe cardiomegaly (CTR, ≥0.75) | 2 (13) | |
| Left chamber dilation | 12 (80) | |
| Pulmonary congestion | 2 (13) | |
| Transthoracic echocardiography | Normal LV function (LVEF, 65%-70%) | 5 (33) |
| Mild LV dysfunction (LVEF, ≥45%-<65%) | 6 (40) | |
| Moderate LV dysfunction (LVEF, ≥30%-<45%) | 3 (20) | |
| Severe LV dysfunction (LVEF <30%) | 1 (7) | |
| No mitral regurgitation | 6 (40) | |
| Grade 1 mitral regurgitation (mild) | 4 (27) | |
| Grade 2 mitral regurgitation (moderate) | 1 (7) | |
| Grade 3 mitral regurgitation (significant) | 1 (7) | |
| Grade 4 mitral regurgitation (severe) | 3 (20) | |
| Cardiac catheterization | LVEDP ≤14 mmHg | 6 (40) |
| LVEDP >14 mmHg | 9 (60) | |
| Origin of coronary artery from posterior aspect of PT wall | 9 (60) | |
| Origin of coronary artery from inner aspect of PT wall | 4 (27) | |
| Origin of coronary artery from lateral aspect of PT wall | 2 (13) |
CTR, cardiothoracic ratio; LV, left ventricle; LVEDP, left ventricular end-diastolic pressure; LVEF, left ventricular ejection fraction; PT, pulmonary trunk.
Figure 2. Characteristic X-ray image of a patient with anomalous origin of left coronary artery from pulmonary trunk. Note the cardiomegaly at the expense of left chambers.
According to echocardiography, 5 patients (33%) had a normal LVEF; left ventricular dysfunction was mild in 6 (40%), moderate in 3 (20%) and severe in only 1 case. In 6 patients (40%), the echocardiogram revealed the absence of mitral regurgitation, whereas in the remaining 9 (60%) it was grade 1 (n=4; 27%), grade 2 (n=1; 7%), grade 3 (n=1; 7%), or grade 4 (n=3; 20%). It should be pointed out that 4 patients (27%) had grade 3 or 4 mitral regurgitation secondary to moderate or severe left ventricular dysfunction. Finally, the catheterization disclosed an increase in LVEDP in 9 cases (60%). The site of origin of the left coronary artery was, in order of frequency, posterior PT wall (60%, 9 cases), inner wall (27%, 4 cases) and lateral wall (13%, 2 cases).
Of the 15 patients studied, only 5 (33%) received an initial diagnosis of ALCAPA. In the remaining 67% (10 cases), a different presumed diagnosis was suggested: mitral regurgitation (33%, 5 cases), cardiomyopathy (17%, 4 cases) and coronary artery fistula (8%, 1 case). Two associated lesions were found: 1 superior sinus venosus atrial septal defect and 1 coronary artery-right ventricular fistula.
Intraoperative ResultsMyocardial protection was achieved by means of moderate hypothermia at a mean temperature of 27°C with antegrade cardioplegia in 7 cases (47%) and combined antegrade and retrograde cardioplegia in 8 cases (53%). Blood cardioplegia was employed in 8 patients and crystalloid cardioplegia in 7. The mean cardiopulmonary bypass time was 115min (range: 77 to 214min) and the mean aortic clamp time, 77min (44 to 138min). The surgical procedure was performed using the variant described for each group: 9 cases (60%) with the technique for origin in posterior wall of PT (Figure 1C), 4 cases (27%) with that for inner wall origin (Figure 1A) and 2 cases with that for origin in the lateral wall (Figure 1B). In every case, the anomalous coronary artery was reimplanted without kinking or tension in the anastomosis. In the 4 patients with grade 3 or 4 mitral regurgitation secondary to moderate or severe ventricular dysfunction (27%) a mitral valve procedure was performed. Three patients (20%) underwent mitral annuloplasty and, in the remaining case, since mitral valve repair was not possible, the decision was made to replace the valve with a no. 23 mechanical prosthesis in supraannular position. As associated procedures, closure of an atrial septal defect with a bovine pericardial patch was performed in 1 case and closure of a coronary-ventricular fistula in another.
Prior to decannulation, follow-up ITEE was carried out in all the patients who had undergone concomitant mitral surgery (4 cases, 27%). In 2 cases, ITEE revealed the absence of mitral regurgitation. Another patient had moderate residual mitral regurgitation, but showed hemodynamic stability that made it possible to wean him from cardiopulmonary bypass and thus no further measures to reevaluate the mitral valve were undertaken. In the patient who required mitral valve replacement, ITEE confirmed that the prosthesis was functioning adequately.
At the time of weaning from cardiopulmonary bypass, arrhythmias were detected in 47% of the patients. Five of them developed ventricular fibrillation and 2 had ventricular tachycardia. At the end of surgery, 3 patients (20%) were treated with open sternum, 2 of them due to hemorrhage that required packing and 1 due to hemodynamic instability when closure was attempted. All of them underwent surgery for sternal closure 2 days later, without complications. Finally, 2 patients (13%) were extubated in the operating room.
Results During the Immediate Postoperative PeriodMortality: the mean age of the patients at the time of correction was 74 months (6.2 years; range, 2 months to 24 years). There was no operative mortality in this series.
Morbidity: morbid events occurred in 9 patients (Table 2). Five patients (33%) required a surgical procedure of some type: 1 patient underwent reexamination due to postoperative hemorrhage and in 4 cases it was necessary to initiate pleural drainage due to effusion. In 3 cases (20%), nitroglycerin was employed because of electrocardiographic evidence of ischemia. Given that the signs of ischemia and secondary heart failure abated, on average, 24h after beginning vasodilator therapy, the most probable etiology of these events was considered to be transient coronary spasm. With the exclusion of the 2 patients (13%) who were extubated in the operating room, the duration of mechanical ventilation was ≤24h in 8 patients (53%), from 1 to 5 days in 1 (7%), from 5 to 7 days in 3 (20%), and ≥7 days in 1 (7%). The mean intensive care stay was 5 days (range: 2 to 11 days; mode, 3 days) and the mean postoperative hospital stay, 10 days (range, 6 to 21 days; mode, 6 days).
Table 2. Immediate Postoperative Morbidity Associated With Anomalous Origin of Left Coronary Artery From Pulmonary Artery With Coronary Reimplantation.
| Complications | Patients, no (%) |
| Low postoperative cardiac output | 9 (38) |
| Pleural effusion | 4 (17) |
| Transient ischemia | 3 (13) |
| Postoperative tachyarrhythmia | 2 (8) |
| Immediate postoperative mediastinal hemorrhage | 1 (4) |
| Postoperative acidosis | 1 (4) |
| Transient complete atrioventricular block | 1 (4) |
| Pericardial effusion without hemodynamic deterioration | 1 (4) |
| Respiratory infection | 1 (4) |
| Transient neurological deficit | 1 (4) |
| Total | 24 (100) |
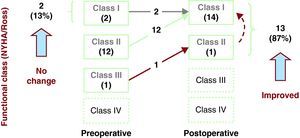
The medium-term follow-up rate in our study was 100%, and the mean duration was 4±3 years (1 to 12 years); all the patients are alive and in functional class I (NYHA/Ross). Figure 3 shows the clinical course of the patients: 13 of them (87%) improved their functional class over the medium term and 2 (13%) showed no changes. The only patient in functional class II was the one who underwent mitral valve annuloplasty concomitantly with coronary reimplantation. Although he was initially asymptomatic with the use of diuretics, his clinical status began to deteriorate and the echocardiogram revealed severe mitral regurgitation and moderate tricuspid regurgitation. He underwent surgery 4 months later for valve replacement by a 23-mm St. Jude HP mechanical aortic prosthesis, which was placed, inverted, in mitral position, in addition to Kay's tricuspid valve repair. The postoperative course was satisfactory and the patient is in functional class I. Another of these patients, despite being asymptomatic, presented signs of anteroseptal and apical ischemia on electrocardiogram. Thus, he underwent a study that included exercise stress test, computed tomographic angiography, coronary angiography, and lipid profile. We reached the conclusion that he had acquired atherosclerotic coronary disease secondary to familial dyslipidemia with no critical coronary lesions. Medical treatment with statins and fibrates was indicated and the disease remains under control to date. At the present time, all the patients are enrolled in a follow-up protocol that includes electrocardiography, spiral computed tomographic angiography, myocardial perfusion scintigraphy, and cardiac catheterization to document the status of their myocardial perfusion.
Figure 3. Changes over the medium term in the functional class of patients who undergo surgical correction of anomalous origin of left coronary artery from pulmonary trunk with coronary reimplantation. NYHA, New York Heart Association.
DISCUSSIONALCAPA was described by Brooks in 1866 and the first clinical and autopsy report was that of Bland et al. in 1933.13, 14, 15, 16 In 1968, Wesselhoeft et al.2 described 2 forms of presentation of ALCAPA: the infantile form, which is more common (82%), and the adult form (18%).2, 3, 4, 5 In our series, the incidences of both types are similar to those reported by Wesselhoeft et al. We consider that the high rate of ALCAPA in adolescents is due to late referral and to the lack of knowledge about this defect. For this reason, the diagnosis is not suspected or is mistaken for other entities such as dilated cardiomyopathy and congenital mitral regurgitation.17, 18, 19
Echocardiography is the standard diagnostic tool since, in addition to demonstrating the existence of the anomaly, it determines ventricular function and reveals the presence of mitral regurgitation and signs of subendocardial fibroelastosis.12, 20, 21 The mechanism of mitral regurgitation in ALCAPA is dysfunction of the papillary muscle (usually posterior) due to ischemia, but it is mainly a consequence of dilation of the mitral annulus secondary to the spheroid configuration adopted by the ischemic left ventricle, a circumstance that produces a defect in mitral leaflet coaptation.22 Thallium-201 myocardial perfusion scintigraphy is highly sensitive and shows hypoenhancement of the ischemic region of the anterolateral wall of left ventricle. On the other hand, spiral computed tomographic angiography is gaining ground in the diagnosis of ALCAPA because of its high sensitivity and specificity. The possibility of 3-dimensional reconstruction is another advantage of this method and it is also a useful alternative in follow-up studies.
Attempts to correct ALCAPA surgically began in 1953 when Potts carried out aortopulmonary anastomosis to increase the oxygen saturation in the PT.23, 24 Mustard described an end-to-end anastomosis of left carotid artery to anomalous left coronary artery.25 The first case of successful surgical management was reported in 1960 by Sabiston et al. the procedure consisted of coronary ligation at the site of the analomous origin.23 In 1968, Meyer et al.26 anastomosed the left subclavian artery to the anomalous left coronary artery. Subsequent attempts to correct ALCAPA focused on establishing a two-coronary system and alternatives such as that of Takeuchi8 and coronary reimplantation5 emerged. The results documented with the Takeuchi procedure have been encouraging since the authors of several series report minimal complications and a low mortality rate. However, complications are recorded during long-term follow-up: aortic regurgitation, supravalvular pulmonary stenosis, and patch obstruction.27 Over the past decade, the cumulative experience with techniques like the Jatene procedure for the transposition of the great arteries has benefited patients with ALCAPA. As surgeons have become increasingly familiar with transfer of the coronary ostium, direct implantation into the aorta has become the procedure of choice in the majority of patients with ALCAPA. The operative mortality associated with this procedure ranges between 0% and 23%, and long-term survival is excellent.28, 29, 30, 31, 32, 33 One of the most extensive experiences is that of Azakie et al.,7 who reported a series of 67 patients, 47 of whom underwent left coronary reimplantation, with a survival rate of 92%. The operative mortality in our series compares favorably with these results. Despite the fact that we do not focus on the description of the late results, we can affirm that the postoperative course over the medium term is satisfactory from the clinical point of view. We have yet to evaluate the functional results of myocardial perfusion, the objective of our follow-up protocol currently underway.
One of the technical aspects that must be considered during the correction of ALCAPA is the position of the anomalous coronary artery. When the origin is on the inner aspect of the PT wall, reimplantation can be carried out with no additional mobilization of the coronary system. The origin of the ALCAPA in posterior or lateral position increases the degree of difficulty of the implantation, given the need for a significant mobilization of the coronary system and the possibility of kinking of the coronary tree during reimplantation. It is not uncommon that the length of the coronary artery is insufficient for the achievement of a tension-free anastomosis. In these cases, techniques for lengthening the coronary artery, such as the pulmonary artery flap,34 have been developed but are not without the risk of ischemic events that can increase the operative mortality rate.
Another controversial aspect in the correction of ALCAPA is related to surgical management in cases of mitral regurgitation. In the majority of the patients with ALCAPA and ventricular dysfunction and/or mitral regurgitation, we can expect recovery after reestablishment of the coronary blood flow. However, it is difficult to determine exactly when the ventricle might recover and when the secondary mitral regurgitation will improve. According to the experience of Azakie et al.,7 following the correction of ALCAPA during the neonatal period or in infancy there is a significant improvement in the LVEF starting 1 year later. Thus, they recommend not performing any procedure associated with the correction of ALCAPA. When ventricular dysfunction is severe and/or mitral regurgitation is grade 3 or 4, valve repair or prosthetic valve replacement is recommended, depending on the anatomy of the mitral valve apparatus. Our group agrees with this approach and for this reason performed an additional mitral valve procedure only in patients with this hemodynamic status. We consider the procedure of choice to be mitral annuloplasty, the results of which should be evaluated in the operating room with ITEE, opting for prosthetic valve replacement in cases of repair failure or of unfavorable mitral anatomy, which occurred in the only patient in our series who received a mechanical prosthesis.
CONCLUSIONSLeft coronary artery reimplantation is a valid surgical option for the management of patients with ALCAPA, with an excellent medium-term survival rate and a low morbidity rate. An in-depth clinical and echocardiographic analysis should be carried out in all patients with heart failure secondary to congenital mitral regurgitation or to cardiomyopathy in order to rule out this coronary anomaly.
CONFLICTS OF INTERESTNone declared.
Received 7 February 2011
Accepted 19 April 2011
Corresponding author: Departamento de Cirugía Cardiaca Pediátrica y Cardiopatías Congénitas, Instituto Nacional de Cardiología Ignacio Chávez, Juan Badiano 1, Edificio H, 6.o piso, Colonia Sección XVI, Delegación Tlalpan, 14080 México D.F., Mexico. pcuricuri001@gmail.com