Keywords
INTRODUCTION
Ventricular septal defect (VSD) is a congenital heart disease that represents 20% of all congenital heart malformations. In many hospitals it is referred to as the most frequent isolated defect, not including bicuspid aortic valve.1,2 It is the second most frequently occurring congenital heart defect, only surpassed by the persistence of arteriosus ductus.3 The incidence of VSD is approximately 1.5 to 3.5 per 1000 newborns.4 Its prevalence in adults is low, fundamentally due spontaneous closing of some defects5 and to that fact that the majority of symptomatic defects are closed surgically during childhood.
There are various types of VSD, some of which are complex.6,7 Currently, the most useful and practical method for classifying VSD is according to the anatomy of the interventricular septum (IVS), which is morphologically made up of 2 parts: the fibrous portion and the muscular portion. The first constitutes the membranous septum; it is relatively small, with a diameter of approximately 5 mm. The second, surrounded by the membranous septum, is divided into 3 areas in order to facilitate study: the inlet, trabecular, and outlet areas. This allows classification of atrial septal defects into musculo-membranous and muscular, in accordance with the type of tissue that makes up its rim. Peri-membranous defects can extend into the inlet, trabecular, or outlet portions of the septum. In defects of the muscle region, the tissue rims are completely muscular in composition and may be located in any part of the septum. Inlet and outlet defects can have muscular or fibromuscular tissue rims. The sub arterial infundibular type (SIVSD) is different from the muscular infundibular type because it has fibromuscular tissue rims and is located under the anterior cusp of the aorta and the right anterior cusp of the pulmonary valve (PV) (Figure 1); these were previously classified as supracristal or doubly-committed.6 The interest is this type of VSD is related to the anatomical substrate, which in some cases causes the development of prolapse and aortic insufficiency (AoI).6,8
Fig. 1. Drawing that shows the interventricular septum seen from the right ventricle. The top of the defect is observed, formed by the aortic and pulmonary sigmoids, which constitute its membranous rim; the muscular rim forms the infundibular septum that delineates the septal defect. The amount of prolapse of the anterior sigmoid is demonstrated via the septal defect (arrow). AoS indicates aortic sigmoid; PV, pulmonary valve; PI, pulmonary infundibular; IVS, interventricular septum.
The presence of an SIVSD must be periodically evaluated clinically and by echocardiography, and its association with prolapse or AoI, or both, tends to be progressive and a cause of later morbidity and mortality.9,10
The aim of this study, carried out at the Instituto Nacional de Cardiología Ignacio Chávez, was to determine the frequency of SIVSD occurrence and its association with aortic valve changes, and to determine the usefulness of transthoracic echocardiography in the diagnosis and follow-up of patients with this type of heart disease, comparing our findings with anatomical specimens for the purpose of correlation.
MATERIALS AND METHODS
Between January, 1998 and December, 2000, 593 cases with a diagnosis of VSD not associated with another congenital heart disease were seen in the external pediatric cardiology practice of the Instituto Nacional de Cardiología; of these, 41 patients had a clinical and echocardiography diagnosis of SIVSD. We reviewed the clinical data and initial echocardiography studies retrospectively, and follow-up echocardiography was performed. We excluded 6 patients from the study who had an uncertain treatment history and incomplete clinical or echocardiography records. The study included 35 patients who had complete echocardiography follow-up; in other words, an echocardiogram had been performed at the time of diagnosis and on at least 1 other occasion during follow-up.
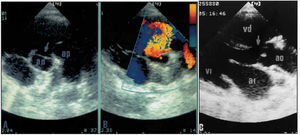
The echocardiography studies included M-mode and 2-dimensional (ECHO-2D) images with conventional cuts, complemented by the various modalities of Doppler echocardiography.10,11 SIVSD was defined by echocardiography showing a continuity fault in the aortic valve visualized in the right upper quadrant in the short parasternal axis (Figure 2A). The shunt via the VSD was evaluated by continuous color echo-Doppler 12 (Figure 2B); on the long parasternal axis we observed the defect that was or was not associated with the continuity fault of the IVS adjacent to the aortic valve (Figure 2C). The size of the SIVSD was calculated in accordance with the ratio of the area to the patient´s body surface. The seriousness of the AoI was measured in accordance with the deceleration gradient determined by continuous Doppler, and in some cases in accordance with the ratio of the diameter of regurgitation flow and the diameter of the outlet tract of the left ventricle (LV). An aortic valve prolapse was considered to exist when on the long parasternal axis aortic valve slipping and deformity of the right coronary valve, or both, was present on the same axis or on the short parasternal axis, or both (Figure 3).
Fig. 2. Echocardiogram of the short axis at the level of the vessels that shows: (A) the septal defect (arrow) and its relationship to the anterior aortic sigmoid and the pulmonary valve, and (B) the shunt as demonstrated by color Doppler. (C) Shows a septal defect on the long parasternal axis. Ao indicates aorta; PA, pulmonary artery
Fig. 3. Echocardiogram of the long parasternal axis where the deformed anterior right coronary sigmoid is observed that prolapses via the septal defect. RA indicates right atrium; LV, left ventricle; AoP, aortic prolapse.
Ten anatomical specimens were analyzed and served as a substrate to establish the correspondence between the type of septal defect and the echocardiography image obtained from equivalent hearts.
The variables studied (age, diameter of the VSD, AoI, body surface, and initial and final follow-up gradient, as well as the length of follow-up) were entered into an Excel database. Statistical analysis was performed with SPSS 9.0 and EPI-INFO 6.0 statistical program, using the Student t test for paired samples, and the difference was considered significant for values P<.05. Age, diameter of the SIVSD, body surface, and initial gradient were compared with the final values. AoI and its progression, at the beginning and the end of the study, were compared with the VSD diameter in a continuous format, and, later, 3 categories were established (small <0.59 cm²; medium 0.60 to 1 cm², and large >1 cm²) that were also compared to the follow-up period.
RESULTS
An infundibular sub arterial ventricular septal defect was present in 6.9% of the study population (41 patients). Six cases were excluded from the study due to the lack of complete echocardiography follow-up. These patients did not present with AoI or AoP, and 1 (2.4%) of them presented with bacterial endocarditis.
Of the 35 cases included in the study, 46% were male. Average age at the time of diagnosis was 5.8 years (range from 1 month to 22.7 years of age). Eight-eight point five percent of patients were admitted because of a cardiac murmur detected on routine examination, and the rest due to signs and symptoms of various degrees of cardiac insufficiency.
Significant changes were observed upon comparison of body surface at initial and final measurement (Table 1); nevertheless, the diameter and gradient of the SIVSD remained practically the same during the periods indicated (Figure 4).
Fig. 4.Graph comparing the initial and final measurements of the SIVSD diameter and gradient during average follow-up (8 years); it can be seen that the defects remained unchanged during this period.
We found 29 cases of small SIVSD, 3 medium size, and 3 large. At the time of diagnosis, 87% of cases (30 patients) did not present with AoI; nevertheless, 11 of these (32%) developed AoI, as could be observed in the last echocardiogram performed in follow-up an average of 8 years later. With respect to those children who had slight AoI at the beginning of the study (3 cases), only 1 progressed to moderate aortic insufficiency (Table 2). Nevertheless, the results show that the majority of cases (63%) did not develop AoI or remained at the same level of AoI that they had at the time of initial diagnosis. When we compared the progression of AoI with follow-up time, we observed that this was practically the same for both the group where AoI did not progress and the group where it did (7.6 years vs 8 years, respectively) (P=.78); similarly, the results showed that the size of the defect did not decrease during the follow-up period. With regard to the size of the SIVSD and the development of AoI, we observed that there was a greater tendency toward the development of insufficiency and progression of AoI, or both, in those patients who had defects that were small in diameter (Table 2).
With regard to AoP, we found that at the time of diagnosis only 1 patient with moderate AoI had AoP. At the time that the cardiogram was performed, we detected AoP in 5 more patients, 2 without AoI and 3 with slight AoI.
Eight of the 35 patients (22.8%) underwent surgery: 3 to correct a large defect, 3 because they presented with significant hemodynamic repercussions with arterial pulmonary hypertension, and 2 as they had moderate AoP and AoI. Surgery was performed at an average patient age of 8.32 years (2.6 to 14.8 years).
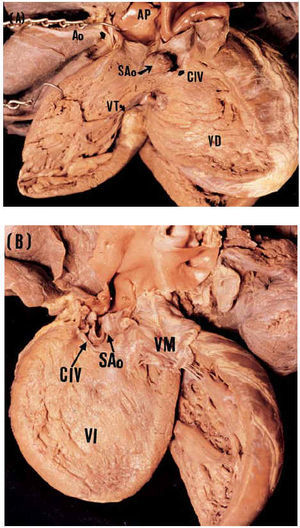
The anatomical specimens represented 10% of the pathology collection of ventricular septal defects of the Instituto Nacional de Cardiología Ignacio Chávez. In this sample, we observed the absence of infundibula septum in the posterosuperior area. The top of the defect was formed by the arterial valves, and the bottom was formed by the anterior arm of the trabecula septomarginalis. Via the defect, the prolapse of the anterior aortic sigmoid was toward the infundibula of the right ventricle (RV) (Figures 5A and 5B). These findings correspond to those observed on echocardiography study. The correlation between this morphological substrate and the echocardiography images was clear and precise (Figures 2, 3, and 5).
Fig. 5. Photographs of the anatomical specimens that show sub arterial atrial septal defect. Internal views of the right ventricle (A) and left ventricle (B) show the location of the defect and its relationship to the aortic and pulmonary sigmoids; the sigmoid prolapse can be observed in both. ASD indicates atrial septal defect; TV, tricuspid valve; MV, mitral valve; Ao, aorta; PA, pulmonary artery; AoS, aortic sigmoid.
DISCUSSION
Of the various types of VSD, SIVSD occurs least frequently; in most series it represents 5% of ventricular septal defects, and the data from our study is similar (6.9% of cases).
This type of septal defect is located under both semilunar valves, causing fibrous continuity between the pulmonary and aortic rings, with the infundibular septum either partially or completely missing, without adequate support for the valves, producing a prolapse of the right coronary valve and on occasion also involving the non-coronary valve,8,13 as observed in the anatomical specimens analyzed.
AoI that appears with an VSD occurs more frequently in cases of the sub arterial infundibular type, developing in approximately 10% of cases,14 and some studies an occurrence rate of up to 20% to 30% has been reported in Asian populations.15 The predisposing anatomical cause of aortic prolapse is the absence of support for the anterior sigmoid, produced by the lack of the infundibular septum immediately below it. The difference in the pressure of the aorta and the pulmonary infundibula (PI) in diastole is what triggers the prolapse, and the consequent insufficiency; for this reason, the risk of complications is increased with regard to the basic hemodynamic disturbance in this type of VSD.16 The progression of AoI becomes a serious complication that under some circumstances dominates the hemodynamic picture. Sometimes, the prolapse of the valve occludes the septal defect, which decreases the arteriovenous short-circuit evidence and improves the cardiac insufficiency data, giving the appearance of a smaller defect, as we observed in one of our cases. If the AoP is very large, it can obstruct the outlet tract of the RV.
We found that in 13% of cases AoI was present at the time of diagnosis, a relatively high percentage that may be explained by the fact that diagnosis was made at school age in the majority of our patients, a period of higher incidence of the development of AoI, which is generally detected between the ages of 5 and 8 years.17 In our series, there was no statistically significant relationship between the size of the SIVSD and the development or seriousness of the AoI, and we only observed a greater tendency to development of AoI in patients with small defects; nevertheless, the number of patients studied limits our results; we assume that if the sample were larger, it is probable that this tendency would be confirmed. We believe that by not having significant repercussions on RV pressure in small VSD, the difference between the PI and the aortic pressures is greater, favoring AoI, as opposed to larger defects where the mechanism is principally one of lack of support.
We observed that the majority of patients (63%) maintained the same level of insufficiency during follow-up; nevertheless, the time elapsed before insufficiency increased was shorter in those cases that evolved from slight to moderate AoI and from moderate to serious AoI than in those patients who did not progress from slight AoI. It is worth noting that by the end of the study 46% of patients had developed some level of AoI.
The characteristic diastolic murmur of AoI cannot always be detected during auscultation, especially when it is slight, and we recommend maintaining a watchful eye over these cases by means of periodic echocardiography studies, which have proven to be an effective non-invasive method for complete evaluation of this type of defect, aortic changes, and follow-up of same.
Contrary to previously published studies, we only found that 31% of patients with AoI presented with AoP. For this type of complaint, before the discovery of AoI, the presence of aortic valve prolapse was specifically investigated.18 Large SIVSD frequently result in the development of arterial pulmonary hypertension (APH) and on rare occasions develop AoP, perhaps due to early surgical intervention.
Aneurysm of the sinus of Valsalva did not present in any of our cases, as the greatest incidence of this complication occurs after the third decade of life.17
There is still controversy with regard to when to initiate surgical treatment, as in the majority of patients the defects are small and do not have hemodynamic repercussions; nevertheless, as has been shown, they also do not close spontaneously. Given that the possibility of developing AoP and AoI increases with age, some authors recommend early surgical treatment, that is to say, before aortic valve changes appear.19,20 This fact is based on studies that have shown that the patients who do not develop aortic valve problems post-surgery are those who did not have either AoP or AoI before surgery; on the other hand, those that had both problems before surgery generally continued to have AoI after surgical intervention, although to a lesser degree than initially.10,21 Early surgical intervention (before 2 to 3 years of age) was only performed if the patient showed signs and symptoms of cardiac insufficiency, was beginning to develop APH, or developed an aortic valve problem. Karpawich22 recommended repair after 5 years of age, a therapy that has better long-term results.21 We believe that surgery should be considered when AoI is presents. Transesophageal echocardiography during surgery allows evaluation of the efficacy of the septal defect correction when AoI is present, and offers sufficient information to ensure the most adequate treatment.23
CONCLUSIONS
The elevated morbidity-mortality rate in patients who have an SIVSD, especially with regard to aortic valve changes, makes frequent clinical and echocardiography evaluations obligatory, especially for those patients with small defects, as these tend to evolve more rapidly into AoI.
Transthoracic echocardiography has become the best non-invasive technique for detecting and following associated aortic lesions. Doppler-echocardiography provides crucial information for establishing the best time for operation.
Finally, it is important to note that surgical treatment must be considered when AoI is present, independently of the hemodynamic repercussions of heart disease, as the development of AoI can complicate the prognosis and survival of the patient.
Correspondence: Dra. Clara A. Vázquez Antona.
Departamento de Ecocardiografía del Instituto Nacional de Cardiología Ignacio Chávez.
Juan Badiano, 1. Col. Sección XVI, Tlalpan C.P. 14080. México, D.F.
E-mail: cvazquezant@yahoo.com.mx