Keywords
INTRODUCTION
Coronary angiography is the reference technique for the diagnosis of coronary disease. However, the majority of acute coronary syndromes involve angiographically non-significant lesions.1 Plaque rupture and consequent thrombus formation is the most common pathogenic mechanism involved in acute coronary syndrome2-4; imaging techniques that can identify these types of lesion are therefore required.
Coronary angiography is also the technique of choice for guiding the implantation of endovascular prostheses and their later monitoring. However, in many situations it has been found wanting in terms of precision, particularly with respect to the detection of complications. These limitations have spurred the search for new intravascular diagnostic imaging techniques. This review discusses that which provides the highest resolution: optical coherence tomography (OCT).
TECHNOLOGY
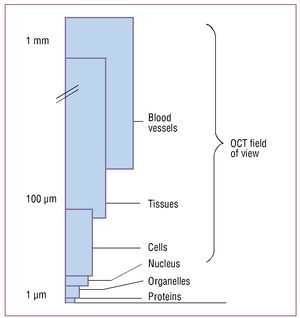
Optical coherence tomography is an interferometric imaging technique that penetrates tissue approximately 2-3 mm and provides axial and lateral resolution on the micrometric scale5,6 (Figure 1).
Figure 1. Field of view in optical coherence tomography. Viewing at the micrometer scale is possible; cells such as macrophages can be identified in the plaque.5
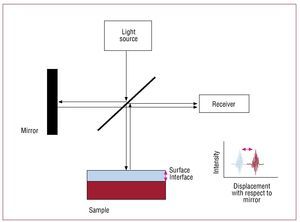
Interferometry, or low coherence interferometry— the basis of OCT—consists of combining the light from different receivers to obtain a high resolution image (Figure 2). In the OCT system, light is divided into 2 rays, a sample ray (directed towards the target) and a reference ray (directed towards a mirror). The combination of the light reflected from the sample and reference rays produces an interference pattern. The areas of the sample that reflect much light produce greater interference than those that reflect no light. Tomographic images are obtained by laterally combining these interferences.
Figure 2.Image capture process in optical coherence tomography (OCT). In OCT, light is divided into 2 beams. One is directed towards the sample, the other towards the mirror. Images are obtained by integrating the characteristics of the light reflected on the receiver.6
Image Capturing Systems
The first commercial OCT system was the M2/ M3 device (LightLab Imaging, Inc., Westford, Massachusetts), which was OCT-time domain-based. Moving through blood vessels at 3 mm/s, this system allowed 20 images to be captured per second (it took 20 s to study a 6 cm-long segment). Images were obtained making use of a 0.019" guidewire, its extreme distal end measuring 0.014". This guidewire consisted of a fiber optic cable inside a cylinder with a mirror at the back. This mirror was displaced from more distal to more proximal positions through the cylinder while performing 360º turns, emitting light and receiving that reflected (ImageWireTM, LightLab Imaging Inc., Westford, Massachusetts). The light of an OCT system is unable to penetrate the blood, so a field free of blood cells is essential. This problem was initially solved using a low pressure over the wire balloon (Helios Occlusion Balloon Catheter, LightLab Imaging Inc., Westford, Massachusetts). This allowed the occlusion of the vessel under study and the distal infusion of isotonic saline at 0.5 mL/h to create a blood cell-free field (Figure 3). Later, Prati et al7 described the possibility of obtaining images without the need for this proximal occlusion balloon, using an infusion of isomolar contrast medium (iodixanol) released from the guidewire.
Figure 3. Method for obtaining images with the LightLab M2/M3 system. The microcatheter is introduced into the coronary artery to be studied. A field free of blood cells is then created via occlusion with a balloon and the distal infusion of saline. The guidewire has a light source and a receiver for exploring the lesion in the distal-proximal direction. Courtesy of LightLab Imaging Inc. (Westford, Massachusetts).
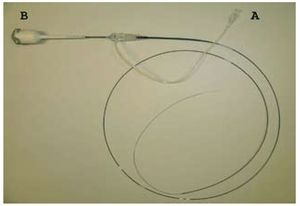
Currently, the C7 XR system, an OCT frequency domain-based system, is marketed in Europe. This uses a laser, making image capture more rapid. Indeed, it provides up to 100 images/s, which allows the studied vessel to be explored at 20 mm/s. The mirror and fiber optics are mounted on a monorail catheter that runs along a conventional guidewire, just as in intravascular ultrasound (IVUS) (Figure 4). Given the speed at which images are obtained (6 cm of an artery can be covered in 3 s), the time that the field has to be free of blood cells is shorter. Thus, an infusion of contrast medium at 4 mL/h, provided via the guidewire, suffices. This system also allows for greater penetration of the light into the artery wall (2-3.5 mm compared to the 1.5-3 mm achieved with the time domain-based OCT system).8
Figure 4. Monorail of the new C7 XR system. No occlusion of the vessel is needed for images to be obtained. The catheter creates a flow of saline and contrast medium that in just a few seconds allows images to be captured. The catheter is connected via point A to a source of saline and contrast medium, and via point B to the console. Courtesy of LightLab Imaging Inc. (Westford, Massachusetts).
Problems and Artifacts
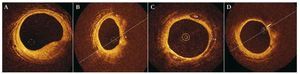
This section discusses the artifacts and most common problems associated with the new frequency domain-based system (Figure 5).9
Figure 5. Most common artifacts with the new C7 XR9 system. A: discontinuity artifact; the rapid movement of the guidewire produces a defect of alignment with the vessel wall, as shown in the example. B: artifact caused by eccentricity of the guidewire; the figure shows the elongation (arrow) of the struts caused by the loss of resolution - illustrating the so-called carrousel phenomenon. C: artifact due to folding; folding (arrow) is sometimes seen at the bifurcations of large vessels; this is characteristic of the new Fourier domain-optical coherence tomography system. D: artifact caused by the presence of blood cells; the arrow signals blood cells inside the vessel; these can be confused with an intravascular thrombus. E: saturation artifact; the arrow signals the artifact produced by the reflection of light by the struts.
Measurement errors due to poor calibration. Before taking OCT measurements, the system must be calibrated, adjusting four marks on the screen to the surface of the catheter. Being just 1% off can lead to a 14% error in the measurements made.
Attenuation in large vessels. The main problem of OCT is the lack of penetration by the light into the artery wall. This can lead to problems in the characterizing of an atherosclerotic plaque and in measuring the artery.
Artifacts caused by the presence of blood cells. Blood in the artery can lead to the vessel wall losing brilliance; this can lead to the confusion of the wall with intravascular thrombotic material.
Artifacts caused by a lack of uniform rotation. If the fiber optic tube does not rotate uniformly during image capture, a consequence of proximal tortuosity of the coronary artery, the vessel wall may appear distorted.
Artifacts caused by discontinuity. Movement of the coronary artery or the guidewire can lead to a lack of vessel wall continuity.
Artifacts of saturation. Light reflected from the struts of a stent produces a signal beyond the system's limit of absorption. The stent is seen distorted, hindering the definition of the surface of the artery under study.
Artifacts of folding. Light reflected outside the system's penetration field, eg, in large vessels or in large lateral branches, causes a characteristic distortion in the form of a "folded" vessel.
Artifacts of eccentricity of the guidewire in the artery. These are usually seen during the analysis of stents. Basically, 2 types of such artifacts may occur, products of the carrousel and sunflower phenomena. The former is caused by differences in the light beam scanning speed in the most distant areas; this leads to a loss of lateral resolution and makes the struts look more elongated. The sunflower phenomenon is caused by a false alignment of the struts around the guidewire that does not coincide with the center of the artery.
APPLICATIONS
Optical coherence tomography is particularly important in the characterization of atherosclerotic plaques and in coronary interventionism. The identification of the culprit plaque is important when attempting revascularization. Important information can be gathered by OCT on the characteristics of a plaque,10-15 allowing it to be defined as either stable or unstable.16
The number of coronary interventions is increasing exponentially, and therefore the number of complications that must be dealt with is also increasing. Optical coherence tomography provides for the adequate visualization of complications related to stent implantation, such as dissection or stent malapposition.17,18 It is also useful for detecting intimal hyperplasia following stent implantation.19
Acute Coronary Syndrome. Identification of the Culprit Plaque
Atherosclerotic plaque rupture and thrombus formation is the mechanism underlying the great majority of acute coronary syndromes.2-4 Some of the characteristics of plaques that predispose them to rupture have been identified. The presence of a large lipid nucleus, a thin fibrous cap or the accumulation of macrophages are among the most commonly cited.2,20,21
Measurement of the Collagen Cap Covering the Plaque
The presence of a thin fibrous cap is a prime characteristic of vulnerable plaques.2,20,21 Optical coherence tomography is the only method currently available with the resolution required for the thickness of this cap to be measured (Figure 6). In a study involving cadavers providing 35 plaques with high lipid contents, Kume et al22 found a strong correlation between the thickness of the collagen cap measured by OCT and histological data (r=0.9; P<.001).
Figure 6. Different types of plaque.10-15 A: plaque with fibrous characteristics; plaques with a homogenous appearance and a high intensity signal are fibrous (F). B: plaque with a high lipid content; the low intensity areas with a poorly defined outline correspond to the lipid nucleus (L). C: plaque with a high calcium content; the areas with a low intensity signal but a well defined border correspond to calcium (Ca). D: the arrow points to the fibrous cap of a thin cap fibroatheroma; below is a poorly defined region of hypodensity corresponding to a lipid nucleus.
In studies performed in vivo, the thickness of the cap recorded by OCT was found to be significantly smaller in patients presenting with acute coronary syndrome than in those presenting with stable angina.15
Optical coherence tomography has allowed the potential stabilization of atherosclerotic plaques through statin treatment to be seen. In a recent observational study involving 40 patients who had suffered a myocardial infarction, it was observed that statin treatment led to a greater increase in the thickness of the fibrous cap (as measured by OCT) than no statin treatment (188 (64%) compared to 117 (39%); P<.01). This increment was more marked in patients in whom the fibrous cap was thinner.23
Plaque Lipid Content
A high plaque lipid content is related to plaque instability. In OCT, plaques with a high lipid content produce a low intensity signal with a poorly defined border10 (Figure 6). There is a tendency to see more of this type of plaque in patients with acute coronary syndrome than in those with stable angina.15
Optical coherence tomography has been shown to possess great sensitivity and specificity with respect to the detection of lipid in plaques.10-14 In all the studies reviewed it was found to be superior to IVUS6 (Table 1).
Intra- and inter-observer variation represent weak points in the determination of the plaque lipid content. In their work on plaque characterization, Manfrini et al13 performed an analysis of plaques with high lipid contents and found a coefficient of interobserver agreement (k) of 0.27 (95% confidence interval [CI] 0.05-0.48). This low k value is probably related to the low penetration of light into the plaques with high fibrous contents, as these authors discussed. In the latter work the sensitivity of the technique in the detection of high lipid content plaques was 45%.
The Significance of Thin Cap Fibroatheroma
Thin cap fibroatheromas (TCFA) are plaques with high lipid contents (2 or more quadrants of the image with lipids) and a thin fibrous cap (<65 µm)15 (Figure 6). The percentage of TCFA in patients with acute coronary syndrome is reported significantly greater than in those with stable angina, and is a common finding when exploring the culprit vessel.15 However, the frequency of lesions with TCFA depends on the artery in question. The proximal segments of the left coronary artery show a higher frequency of TCFA compared to the distal segment. In the right coronary artery, in contrast, the distribution of TCFA is similar throughout the vessel.24
In a study involving 54 lesions, positive remodeling (according to the IVUS remodeling index) compared to negative remodeling was associated with a higher proportion of thin cap fibroatheroma (TCAF) as determined by OCT.
Plaque Calcium Content
Plaques with calcium contents are seen in OCT as regions of low intensity with a well defined border10 (Figure 6). This border definition differentiates them from plaques with a high lipid content. The proportion of calcium in the plaques of patients presenting with acute coronary syndrome is significantly higher than in those presenting with stable ischemic cardiomyopathy.15
The majority of publications indicate that the sensitivity and specificity of detecting calcium by OCT are similar to those reported for IVUS10-14,26 (Table 1). The detection of calcium is of particular importance when choosing a revascularization technique; lesions with high calcium contents are associated with higher rates of stent malapposition.27 Manfrini et al13 showed that the k index was slightly higher for high calcium-content plaques than for plaques with high lipid contents (k=0.4; 95% CI, 0.17-0.63).
Plaque Macrophage Content
The macrophage content of atherosclerotic plaques is directly related to their instability.2,20,21
Optical coherence tomography allows both the density and distribution of macrophages in plaques to be determined.28
Tearney et al28 report a positive correlation between histological and OCT data with respect to the density of macrophages on the fibrous cap of the plaque (r=0.84; P<.0001). The OCT system measures the intensity of the light reflected from the plaque. Plaques that are very heterogeneous in terms of their refractive index give a high intensity signal.
In an in vivo study involving 119 plaques rich in lipid, a significantly higher concentration of macrophages was seen in patients with unstable angina and ST-elevated myocardial infarction (STEMI) (5.86 [1.148%] and 5.54 [2.01%] respectively) compared to patients with stable angina (4.14 [1.81%]). In the same study, larger concentrations of macrophages were seen in the proximity of the plaque rupture in comparison with more distant areas (6.95 [1.6%] vs 5.29 [1.17%]; P=.002).29 No significant differences were seen in the density of macrophages between diabetic and non-diabetic patients (5.94% vs 5.94%; P=0.37).30
Plaque Rupture
The rupture of a plaque allows the culprit vessel to be identified.2-4 Using images obtained by OCT, the rupture of an atherosclerotic plaque has been defined as the discontinuity of the fibrous cap and the formation of a cavity in the plaque. The loss of endothelial continuity with no cavity formation has been termed erosion.31
In a study involving 41 patients with ST-elevated acute coronary syndrome (STEACS), Kubo et al32 observed that in 73% it was possible to identify a plaque rupture, a percentage significantly higher than that returned by IVUS (40%; P=.009) or angioscopy (47%; P=.035). These data contrast with those obtained in an earlier study by Jang et al,15 who identified ruptured plaques in 25% of their patients with STEMI. In the latter work no differences were detected between patients grouped by the type of syndrome with which they presented. A possible explanation for the differences between the results of these studies might lie in the different time elapsed between clinical presentation and image capture (3.8 [1] h in Kubo et al and 4.6 [5.3] days in Jang et al).
A smaller percentage of plaque rupture was reported in a recent cross-sectional study in patients treated with statins who had good lipid control.33 Plaque rupture was detected in 39.6% of the patients with STEMI, in 35.4% of those with stable angina, and in 25% of those with unstable angina.
Thrombotic Content
Thrombotic material is always seen with OCT in patients who present with STEMI; with IVUS, however, the detection rate is significantly lower.32 In the work of Kubo et al32 mentioned above, OCT detected thrombotic material in 100% of patients presenting with acute myocardial infarction, whereas IVUS only found this in 33%. The resolution of OCT even allows a thrombus to be characterized as red or white. Red thrombi, which have a high fiber content, attenuate the signal more than do white thrombi since the light cannot penetrate them. This at least appears to be deducible from the work of Kume et al,34 which was performed with cadavers. These findings were confirmed in in vivo work in experimental animals.35 However, the study by Kume et al only included patients selected for their high probability of showing thrombi. Some authors indicate distinguishing between a thrombus and other abnormal intraluminal phenomena can be difficult, especially when the operator is unaware of the patient's clinical condition.9 In addition, despite the high resolution offered by OCT, the presence of residual blood in the vessel during a procedure can lead to an artifact that might be erroneously identified as a thrombus.9
Coronary Interventionism
In comparison with other endovascular diagnostic techniques used in percutaneaous coronary interventionism, OCT can provide a great deal of information.36 Its high resolution allows the diameter and the luminal area of vessels to be properly measured, information of great use when choosing an endovascular prosthesis. In addition, it is more sensitive than IVUS in the detection of dissections, stent malapposition or small plaque prolapses. The clinical and prognostic importance of findings using IVUS is a matter of debate. While some IVUS studies have reported adverse event rates to be similar in patients with and without acute stent malapposition or plaque prolapse,37-39 others report a high prevalence of post stent-implantation anomalies in patients with acute stent thrombosis.40 The prognostic implications of these findings when detected by OCT is currently unknown; medium to long term studies are needed in this regard.
Measurement of the Luminal Area
In a recent study involving experimental animals, Suzuki et al41 indicated a strong correlation between measurements of the luminal area by OCT and histological data (r=0.98; P<.001) — stronger than that observed between IVUS/histological measurements (r=0.803; P<.001). Observer variation in this work was reported lower than that seen with IVUS (3.7% for OCT vs 4.3% for IVUS). A recent paper published in this journal42 provides in vivo data regarding the differences between IVUS and OCT measurements of the luminal area, with IVUS providing larger figures than OCT. Differences are also seen when using different OCT techniques; the values for the occlusive technique are slightly smaller than those returned by the non-occlusive technique. It should be remembered that the current cut-off values for use in taking clinical decisions were established using IVUS and are not applicable when using OCT.
When using OCT, which requires semi-manual calibration, it is of great importance that the image be properly calibrated. Being out by just 1% can lead to errors of 12-14% in area measurements.9 In addition, small changes in the calibration can amplify any distortion of the vessel outline, leading to the erroneous interpretation of images.
Complications of the Implant: Inadequate Stent Apposition
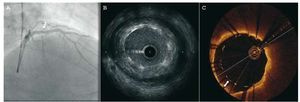
The high resolution of OCT has allowed different degrees of stent apposition to be characterized43 (Figure 7). The frequency of detection of stent malapposition is greater with OCT than with IVUS. In a recent in vivo study by Kubo et al,44 the detection rate with OCT was 47% in a sample of 55 patients, while with IVUS it was only 18% (P<.001). Similar results were reported by Bouma et al45 five years earlier in a study involving experimental animals. Optical coherence tomography can detect a lack of endothelialization in malapposed stents. This might be a determining factor in late stent thrombosis,46,47 although this was not documented in earlier prospective studies involving OCT.
Figure 7. Angiographic images obtained by intravascular ultrasound (IVUS) and optical coherence tomography (OCT) following the implantation of a stent in the anterior descending artery. The angiographic image (A) suggests an optimum result (arrow). The IVUS (B) image shows an area with stent malapposition, which is seen with more definition by OCT (C). The arrow signals the distance between the struts and the vessel wall.
Optical coherence tomography has been reported useful in characterizing stent apposition in segments in which two stents overlap.48 Since the introduction of drug-eluting stents, the length of the segments treated has continued to increase, and in many interventions it has become necessary to overlap stents. The presence of overlapped stents might condition later endothelialization and suboptimal stent expansion, favoring thrombosis.
The use of OCT has shown that up to 40% of the struts in the overlapping segments of 2 stents can be malappositioned. The clinical significance of this, however, is yet to be determined.
One of the limitations of OCT in the diagnosis of stent malapposition is its scant capacity to assess the remodeling of the vessel, a problem of low depth resolution. This characteristic of current OCT systems does not allow the mechanisms or consequences of stent malapposition to be adequately examined.43
Complications of Implantation: Dissection
Kubo et al44 reported that OCT detected dissections in 40% of their patients, while IVUS detected these problems in just 16%. The problems this type of complication may bring in the long term remain unknown. More studies are needed to determine whether any action is required with respect to these complications once detected.
Detection of Neointima
Optical coherence tomography allows the detection and measurement of intimal thickening following stent implantation.19,49,50 Prati et al51 have documented a linear correlation between the measurements obtained with OCT and histological values (R2=0.726; P<.0001). In addition, intra- and inter-observer reproducibility were R2=0.9 and R2=0.8, respectively. The detection of the post-implantation intima by OCT and IVUS has been compared. After a median follow-up time of 6 months, Matsumoto et al52 examined the reaction of the intima by OCT in a study involving 57 drug-eluting stents and found that 64% of the struts had become covered with an intima <100 µm thick (below the resolution of IVUS). A number of publications are now available that describe the process of endothelialization in different types of stent.53,54 Chen et al.5 recently reported observing greater endothelialization following the implantation of non-drug-eluting stents compared to their drug-eluting counterparts. The maximum intimal hyperplasia for non-drug-eluting stents assessed at 5-10 months was 0.36 mm, while for drug-eluting stents assessed at 6-12 months it was 0.07 mm. The OCT system is now being used to assess endothelial generation associated with absorbable stents.56-58
Unfortunately, OCT cannot presently distinguish well the type of tissue covering a stent (eg, endothelium, smooth cell masses, extracellular matrix, or thrombin).9,43 Future studies that include correlations with histopathological information might allow normal endothelialization to be distinguished. Currently, the resolution of OCT is insufficient to allow the detection of thicknesses <20 µm, which prevents any clear distinction being made between the absence of endothelialization and the presence of some 3-5 layers of cells.9
The detection of the endothelialization of drug-eluting stents by OCT could help in the decision to suspend double anti-aggregation treatment in some patients. However, the limitations mentioned above require that new clinical studies be undertaken to demonstrate that such a course of action is safe.Clinical Studies
The possible applications of OCT mean it is being used more and more in different clinical studies for the assessment of results achieved and/ or complications encountered. Recent studies have shown its safety in routine clinical practice,59 with low major complication rates reported, eg, ventricular fibrillation 1.1%, air embolism (0.6%), and coronary dissection (0.2%). It is also being used to assess patients who have undergone prior coronary interventions. The technique also allows information to be gathered on the possible mechanism of restenosis in patients revascularized using a stent. As mentioned above, stent malapposition can be detected by OCT, although the clinical significance of such a finding remains unclear. Some IVUS data suggest that incomplete apposition to the arterial wall might determine the onset of thrombosis.60 The more precise data provided by OCT might provide the key to correctly interpreting this complication. It has been shown that patients who receive a drug-eluting stent during primary angioplasty have a higher rate of malapposition than those who are subjected to intervention because of stable or unstable angina.61 It has been proposed that the dissolution of the thrombus or the elastic recoil of the stent might be the cause of such findings. The rate of incomplete apposition according to the type of stent implanted (drug-eluting or non-drug-eluting)62 or the type of mesh used to make the stent has also been examined.63
The results of several studies examining the coverings of different types of drug-eluting stent by OCT have recently been published. For example, in the OCT substudy of the LEADERS (Limus Eluted From A Durable Versus Erodable Stent Coating) study it was found that, at 9 months, stents with a bioabsorbable polymer had a thicker coating than conventional drug-eluting stents.64 In the ODESSA (OTC for Drug-Eluting Stent Safety) study, patients with long lesions that required multiple overlapping stents were recruited and randomly assigned to receive different types of stent; the coating was then examined at 6 months. The preliminary results show there to be differences in the thickness of the coating depending on the stent type and vessel segment analyzed (overlapping zone or non-overlapping zone).65 Finally, the medical community is awaiting the definitive results of the OCT substudy of the HORIZONS (Harmonizing Outcomes With Revascularization and Stents in AMI) study. In this work, which involved 117 patients treated with primary angioplasty for STEACS, OCT was used at 13 (1) months after the implantation of a drug-eluting stent to examine the latter's coating and apposition, and to check for the presence of any abnormal tissue responses.66
Another area where OCT is beginning to be used is the assessment of patients with saphenous vein graft bypass prior to angioplasty. The system allows much better definition of the type of tissue generated in the saphenous vein and can detect mobile elements inside the bypass (which theoretically increase the risk of embolism).65
COMPARISON WITH OTHER ENDOVASCULAR IMAGING TECHNIQUES
Many invasive and non-invasive imaging techniques have been developed in recent years67-69 (Table 2). The safety and ease of use of IVUS has made it the most employed technique in endovascular imaging. The main advantage of IVUS over OCT is its greater penetration into the tissue, which allows it to measure more precisely the size of the vessel and area of the plaque under study.26
Intravascular ultrasound-virtual histology (IVUSVH), a new type of IVUS, allows for the quantitative analysis of plaque components.12 However, OCT is better for measuring the collagen cap of plaques32,70 and for determining the macrophage content, and it provides higher resolution images than any type of IVUS. Owing to its low resolution (>100 µm), IVUSVH simply cannot distinguish the thin fibrous cap. However, while OCT may have better resolution (10-20 µm), its penetrance is low (<2 mm). Thus, OCT can detect the fibrous cap of a plaque but cannot detect deep-lying lipids. In combination, however, these techniques could allow for the precise diagnosis of TCFA to be made, as reported in a recent study.71
The only other imaging techniques to have provided significant results are angioscopy and thermography. Angioscopy, however, cannot offer any morphological information about plaques.72 In the identification of vulnerable plaques, OCT is therefore much superior. Angioscopy has been used to assess the endothelialization of different types of stent. The literature includes two studies with a small number of patients in which this was assessed. Although no direct comparison was made between OCT and angioscopy, the former allows the thickness of the intima to be measured more precisely; angioscopy relies on the greater or lesser degree of visualization of the struts.73,74
Thermography provides functional information, but no morphological information. No comparisons have been made between this technique and OCT, but given its characteristics they might be complementary.
CONCLUSIONS
Optical coherence tomography is the newest technique to be used in cardiac catheterization. Its high resolution, which was originally used to allow the characterization of atherosclerotic plaques, has recently been employed in coronary interventionism, in which it may have a role to play with respect to the implantation of coronary endoprostheses and the study of any complications that might arise. Certainly, its development for the study of the endothelialization of drug-eluting stents would seem to be important.
Compared to other systems, OCT would appear to offer advantages in the intravascular diagnostic setting, and is an imaging technique with a bright future.
ABBREVIATIONS
IVUS: intravascular ultrasound
IVUS-VH: intravascular ultrasound-virtual histology
OCT: optical coherence tomography
STEACS: ST-elevated acute coronary syndrome
TCFA: thin cap fibroatheroma
Dr Jesús Herrero-Garibi thanks Medtronic Ibérica, S.A. and the Hospital Universitario de Salamanca for funding his stay at the Massachusetts General Hospital in Boston.
Correspondence: Dr I-K. Jang,
Cardiology Division. Massachusetts General Hospital, Gray/Bigelow 800. 55 Fruit Street. Boston, MA 02114. USA
E-mail: ijang@partners.org