Stent thrombosis (ST) is a rare but potentially serious complication. Optical coherence tomography (OCT) provides high-resolution images and additional information to angiography in the study of this event.
MethodsProspective study of patients with ST undergoing reintervention with OCT imaging.
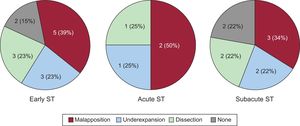
ResultsThe study included a total of 40 consecutive patients with ST. Mean age was 69 ± 13 years and 83% were male. Early ST (≤ 30 days) was observed in 16 patients and late ST (> 30 days) in 24 patients. Stent thrombosis occurred in 17 bare-metal stents and 23 drug-eluting stents. In 34 patients (85%), adequate OCT images were obtained at the time of the ST. The predominant mechanism in early ST was stent malapposition (39%). In late ST, high frequencies of uncovered (46%) and malapposed struts (17%) were observed, especially in patients with drug-eluting stents. Furthermore, the presence of neoatherosclerosis was very high (67%) in patients with late ST. After intervention, improvements were observed in malapposition length and the amount of residual thrombus.
ConclusionsOCT allows identification of the underlying mechanisms potentially involved in ST. This imaging modality is helpful in guiding reintervention in these patients, which improves the area and length of malapposition, as well as the maximal residual thrombus area.
Keywords
Stent thrombosis (ST) is a rare but serious complication of interventional cardiology, with an incidence of about 1%.1–5 Even with appropriate early treatment, this event is associated with high morbidity and mortality.1–5 There are 4 categories of ST: acute (< 24hours), subacute (from 24hours to ≤ 30 days), late (from 30 days to 1 year), and very late (> 1 year).3–5 Its pathophysiological mechanism is highly variable.1–5 Previous studies have identified different pathological mechanisms, depending on whether the ST occurs in a bare-metal stent (BMS) or a drug-eluting stent (DES), in addition to the time from stenting to the clinical event.3–5 Several studies have determined that the main causes of early ST (acute and subacute, ≤ 30 days) are stent underexpansion and/or malapposition. At the same time, neoatherosclerosis with plaque rupture could play a crucial role in very late ST.3–5
Conventional angiography is insufficient to determine the underlying intimal factors leading to ST. Various researchers have used intracoronary ultrasound to obtain additional morphological information and detect mechanical problems in these patients.4 Most of these problems cannot be detected with angiography.4–9 More recently, optical coherence tomography (OCT), a technique with better resolution and image quality, has enabled much more accurate analysis of the possible causes of ST. However, due to the rarity of this complication, few data are available.10,11
The aim of this work was to study the use of OCT in patients who experience ST by analyzing all data that could help to identify the anatomical characteristics potentially related to this alarming complication.
METHODSPatients and InterventionsThe present study enrolled all consecutive patients admitted to our center with a diagnosis of ST from October 2013 to March 2016. During this time, a prospective and systematic protocol was applied that included OCT scans before and after the procedure. The overriding concern was to ensure patient safety, and OCT was only performed when permitted by patients’ clinical and hemodynamic status. Patients with a TIMI (Thrombolysis in Myocardial Infarction) flow of 0 to 1 after intracoronary administration of nitroglycerin and angioplasty guidewire crossing systematically underwent thrombus aspiration with a 6-Fr device. If this maneuver failed to achieve an adequate anterograde flow, a small-diameter balloon (≤ 2mm) was advanced to the occluded segment and inflated at low pressures.
No specific criteria were established to guide the interventions, and the treatment used in each patient was chosen by the interventional cardiologist.
Stent thromboses were diagnosed according to the criteria of the Academic Research Consortium.12 All included patients met the angiographic criteria for “definite” ST and were treated in accordance with the clinical practice guidelines for revascularization.1
All participants were explained the study protocol and signed informed consent. The study was approved by the ethics committee of the hospital.
Optical Coherence TomographyAll OCT studies were performed with an OCT Frequency Domain system (Dragon Fly, Light Lab, St Jude Medical; St Paul, Minnesota, United States) via a nonocclusive technique by advancing the catheter 10mm distal to the stent.
All OCT sequences obtained were first assessed to confirm that their quality was sufficient for subsequent analyses. Metallic struts were visible as brilliant structures with high signal intensity and dorsal shadowing. Reference vessel measurements were taken 5 to 10mm from the stent edges; effort was made to select the least affected section.4,13 Subsequently, a quantitative morphometric analysis was performed, with measurements of the cross-sectional area and diameter. The luminal and stent areas were measured throughout the length of the stent at 1-mm intervals. The minimal lumen area and minimal stent area were determined before and after the intervention. The mean reference area was calculated as the mean of the proximal and distal reference areas. If the pullback did not include images of the proximal and distal segments, the visible reference area alone was used. The stent expansion index (SEI) was calculated as the minimal stent area divided by the mean reference area.13
Thrombus presence, strut coverage and malapposition, and neoatherosclerosis presence were assessed in all cross-sections.13 All sections with thrombi blocking adequate study of at least 2 quadrants of the vessel circumference were excluded. The maximal thrombus area was studied in the cross-section showing the greatest amount of intraluminal thrombi. Struts were considered to be uncovered (classically considered nonendothelialized) if any part of the strut appeared to be directly exposed to the vessel lumen, and the number of cross-sections showing at least 1 uncovered stent strut was counted. Struts were considered malapposed when the axial distance between the surface of the stent and the surface of the vessel was greater than the thickness of the strut. An “image to image” longitudinal analysis was performed that counted the number of sections with at least 1 stent strut showing poor apposition, and the maximal area and diameter of the malapposition was determined. At the same time, the maximal malapposition length was obtained from a longitudinal view of the image.13–15
Neointimal atherosclerotic changes (neoatherosclerosis) were defined as the presence of: a) lipid tissue within the stent (defined as a region with low signal and diffuse edges that caused sufficient signal attenuation to shadow stent struts); b) fibroatheroma (both thin-cap [≤ 65μm] and thick-cap [> 65μm]); or c) neointimal calcification.
Given that some previous studies indicated that the pathophysiological mechanism underlying ST varies according to the time since implantation,3–5 the sample was divided into 2 main groups: early ST (≤ 30 days) and late ST (> 30 days). In addition, the most likely underlying cause of the ST was also evaluated, which was generally associated with the region of most thrombosis. The possible ST mechanisms were the following: stent malapposition, severe stent underexpansion, neoatherosclerosis, proximal or distal edge dissection, and plaque rupture in the coronary segment adjacent to the stent. Another potential cause of late ST was a lack of strut coverage.
Statistical AnalysisContinuous variables are presented as the mean ± standard deviation or as the median [interquartile range], depending on their distribution. Normality was determined using the Kolmogorov-Smirnov test. The Student t test or median test (continuous variables) and the chi-square test, Fisher exact test, or McNemar test (qualitative variables) were used to determine differences between groups. P < .05 was considered statistically significant.
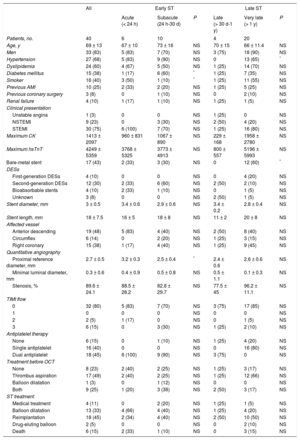
RESULTSBaseline CharacteristicsPatients’ baseline characteristics are summarized in Table 1. The study included 40 consecutive patients diagnosed with definite ST. The mean age was 69 years, 83% were men, and there was a high prevalence of cardiovascular risk factors (Table 1). Most of the thrombosed stents were DESs (58%). The most frequent clinical presentation was ST-segment elevation acute myocardial infarction (n = 30; 75%), with TIMI flow 0 (n = 32; 80%), and the most frequently affected vessel was the anterior descending artery (n = 19; 48%). No significant differences in clinical characteristics were seen between patients with early ST (≤ 30 days) and those with late ST (> 30 days), except for a higher frequency of hypertensive patients in the early ST group. The most frequently used final treatments of the ST were implantation of a new stent (n = 18; 45%) and balloon dilatation alone (n = 13; 33%) (Table 1).
Baseline and Angiographic Characteristics of Patients With Stent Thrombosis
| All | Early ST | Late ST | |||||
|---|---|---|---|---|---|---|---|
| Acute (< 24 h) | Subacute (24 h-30 d) | P | Late (> 30 d-1 y) | Very late (> 1 y) | P | ||
| Patients, no. | 40 | 6 | 10 | 4 | 20 | ||
| Age, y | 69 ± 13 | 67 ± 10 | 73 ± 16 | NS | 70 ± 15 | 66 ± 11.4 | NS |
| Men | 33 (83) | 5 (83) | 7 (70) | NS | 3 (75) | 18 (90) | NS |
| Hypertension | 27 (68) | 5 (83) | 9 (90) | NS | 0 | 13 (65) | * |
| Dyslipidemia | 24 (60) | 4 (67) | 5 (50) | NS | 1 (25) | 14 (70) | NS |
| Diabetes mellitus | 15 (38) | 1 (17) | 6 (60) | * | 1 (25) | 7 (35) | NS |
| Smoker | 16 (40) | 3 (50) | 1 (10) | * | 1 (25) | 11 (55) | NS |
| Previous AMI | 10 (25) | 2 (33) | 2 (20) | NS | 1 (25) | 5 (25) | NS |
| Previous coronary surgery | 3 (8) | 0 | 1 (10) | NS | 0 | 2 (10) | NS |
| Renal failure | 4 (10) | 1 (17) | 1 (10) | NS | 1 (25) | 1 (5) | NS |
| Clinical presentation | |||||||
| Unstable angina | 1 (3) | 0 | 0 | NS | 1 (25) | 0 | NS |
| NSTEMI | 9 (23) | 0 | 3 (30) | NS | 2 (50) | 4 (20) | NS |
| STEMI | 30 (75) | 6 (100) | 7 (70) | NS | 1 (25) | 16 (80) | NS |
| Maximum CK | 1413 ± 2097 | 960 ± 831 | 1067 ± 890 | NS | 229 ± 168 | 1958 ± 2780 | NS |
| Maximum hsTnT | 4249 ± 5359 | 3768 ± 5325 | 3773 ± 4913 | NS | 800 ± 557 | 5196 ± 5993 | NS |
| Bare-metal stent | 17 (43) | 2 (33) | 3 (30) | NS | 0 | 12 (60) | * |
| DESs | |||||||
| First-generation DESs | 4 (10) | 0 | 0 | NS | 0 | 4 (20) | NS |
| Second-generation DESs | 12 (30) | 2 (33) | 6 (60) | NS | 2 (50) | 2 (10) | NS |
| Bioabsorbable stents | 4 (10) | 2 (33) | 1 (10) | NS | 0 | 1 (5) | NS |
| Unknown | 3 (8) | 0 | 0 | NS | 2 (50) | 1 (5) | NS |
| Stent diameter, mm | 3 ± 0.5 | 3.4 ± 0.6 | 2.9 ± 0.6 | NS | 3.4 ± 0.2 | 2.8 ± 0.4 | NS |
| Stent length, mm | 18 ± 7.5 | 16 ± 5 | 18 ± 8 | NS | 11 ± 2 | 20 ± 8 | NS |
| Affected vessel | |||||||
| Anterior descending | 19 (48) | 5 (83) | 4 (40) | NS | 2 (50) | 8 (40) | NS |
| Circumflex | 6 (14) | 0 | 2 (20) | NS | 1 (25) | 3 (15) | NS |
| Right coronary | 15 (38) | 1 (17) | 4 (40) | NS | 1 (25) | 9 (45) | NS |
| Quantitative angiography | |||||||
| Proximal reference diameter, mm | 2.7 ± 0.5 | 3.2 ± 0.3 | 2.5 ± 0.4 | * | 2.4 ± 0.6 | 2.6 ± 0.6 | NS |
| Minimal luminal diameter, mm | 0.3 ± 0.6 | 0.4 ± 0.9 | 0.5 ± 0.8 | NS | 0.5 ± 1.1 | 0.1 ± 0.3 | NS |
| Stenosis, % | 89.6 ± 24.1 | 88.5 ± 28.2 | 82.8 ± 29.7 | NS | 77.5 ± 45 | 96.2 ± 11.1 | NS |
| TIMI flow | |||||||
| 0 | 32 (80) | 5 (83) | 7 (70) | NS | 3 (75) | 17 (85) | NS |
| 1 | 0 | 0 | 0 | NS | 0 | 0 | NS |
| 2 | 2 (5) | 1 (17) | 0 | NS | 0 | 1 (5) | NS |
| 3 | 6 (15) | 0 | 3 (30) | NS | 1 (25) | 2 (10) | NS |
| Antiplatelet therapy | |||||||
| None | 6 (15) | 0 | 1 (10) | NS | 1 (25) | 4 (20) | NS |
| Single antiplatelet | 16 (40) | 0 | 0 | NS | 0 | 16 (80) | NS |
| Dual antiplatelet | 18 (45) | 6 (100) | 9 (90) | NS | 3 (75) | 0 | NS |
| Treatment before OCT | |||||||
| None | 8 (23) | 2 (40) | 2 (25) | NS | 1 (25) | 3 (17) | NS |
| Thrombus aspiration | 17 (49) | 2 (40) | 2 (25) | NS | 1 (25) | 12 (66) | NS |
| Balloon dilatation | 1 (3) | 0 | 1 (12) | NS | 0 | 0 | NS |
| Both | 9 (25) | 1 (20) | 3 (38) | NS | 2 (50) | 3 (17) | NS |
| ST treatment | |||||||
| Medical treatment | 4 (11) | 0 | 2 (20) | NS | 1 (25) | 1 (5) | NS |
| Balloon dilatation | 13 (33) | 4 (66) | 4 (40) | NS | 1 (25) | 4 (20) | NS |
| Reimplantation | 18 (45) | 2 (34) | 4 (40) | NS | 2 (50) | 10 (50) | NS |
| Drug-eluting balloon | 2 (5) | 0 | 0 | NS | 0 | 2 (10) | NS |
| Death | 6 (15) | 2 (33) | 1 (10) | NS | 0 | 3 (15) | NS |
AMI, acute myocardial infarction; CK, creatine kinase; DES, drug-eluting stent; hsTnT, high-sensitivity troponin T; NS, not significant; NSTEMI, non—ST-segment elevation myocardial infarction; OCT, optical coherence tomography; ST, stent thrombosis; STEMI, ST-segment elevation myocardial infarction; TIMI, Thrombolysis in Myocardial Infarction.
Unless otherwise indicated, the data represent No. (%) or mean ± standard deviation.
Baseline OCT was performed in 35 patients admitted with ST (14 with early ST and 21 with late ST). In 1 patient with early ST, the quality of the baseline OCT images was insufficient for their interpretation due to a blood artifact and considerable thrombosis. Thus, the results of 34 patients were finally analyzed. In 77% of patients, thrombus aspiration and/or balloon predilatation were required to achieve a TIMI distal flow score of 2 and obtain OCT images of sufficient quality. Representative OCT images from patients with ST are shown in Figure 1.
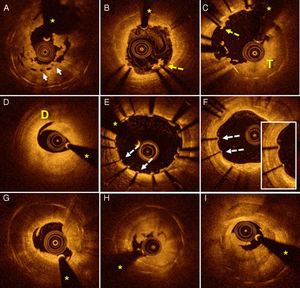
Stent thrombosis assessed using OCT. A: severe underexpansion of a bioabsorbable vascular device (arrows). B: areas of malapposition (dotted arrow) with associated thrombus. C: extensive areas of malapposition (dotted arrow) with a large thrombus burden (T). D: marked dissection (D) at the distal edge of a device implanted several hours before. E: extensive areas with coverage deficiency (dotted arrows) and other regions with slight malapposition. F: patient with very late ST whose OCT shows uncovered struts (dotted arrows). G-I: patients with very late ST with images of neoatherosclerosis with distal shadowing obscuring the stent struts; in addition, plaque rupture images with associated thrombus. Asterisks indicate shadowing caused by a wire artifact. OCT, optical coherence tomography; ST, stent thrombosis.
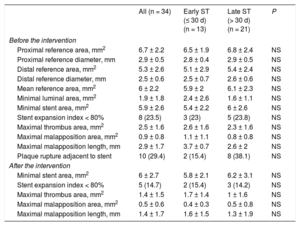
The data obtained with OCT are reported in Table 2. Before treatment, the mean reference area was 6.0 ± 2.2mm2 and the minimal stent area was 5.9 ± 2.6mm2. After treatment, there was a slight rise in the minimal stent area (6.0 ± 2.7mm2; P = .59), an increase that was somewhat higher in the subgroup of patients with early ST (from 5.4 ± 2.2mm2 to 5.8 ± 2.1mm2; P = .29) (Table 2). The number of patients with an SEI < 0.8 also decreased, again without statistical significance. An SEI < 0.8 was seen in 24% of patients (n = 8) before the treatment vs 15% (n = 5) after the intervention. In addition, there was a trend for a decreased percentage of cross-sections showing malapposed struts after the intervention (6.8% ± 11.9% before intervention vs 4.2% ± 7.0% after; P = .2) and a decreased malapposition area (0.87 ± 0.80mm2 vs 0.45 ± 0.60mm2; P = .15). There was also a significant decrease in the malapposition length (2.93 ± 1.70mm vs 1.43 ± 1.70mm; P = .015).
Analysis of Lesions by Optical Coherence Tomography Before and After Intervention for All Stent Thromboses
| All (n = 34) | Early ST (≤ 30 d) (n = 13) | Late ST (> 30 d) (n = 21) | P | |
|---|---|---|---|---|
| Before the intervention | ||||
| Proximal reference area, mm2 | 6.7 ± 2.2 | 6.5 ± 1.9 | 6.8 ± 2.4 | NS |
| Proximal reference diameter, mm | 2.9 ± 0.5 | 2.8 ± 0.4 | 2.9 ± 0.5 | NS |
| Distal reference area, mm2 | 5.3 ± 2.6 | 5.1 ± 2.9 | 5.4 ± 2.4 | NS |
| Distal reference diameter, mm | 2.5 ± 0.6 | 2.5 ± 0.7 | 2.6 ± 0.6 | NS |
| Mean reference area, mm2 | 6 ± 2.2 | 5.9 ± 2 | 6.1 ± 2.3 | NS |
| Minimal luminal area, mm2 | 1.9 ± 1.8 | 2.4 ± 2.6 | 1.6 ± 1.1 | NS |
| Minimal stent area, mm2 | 5.9 ± 2.6 | 5.4 ± 2.2 | 6 ± 2.6 | NS |
| Stent expansion index < 80% | 8 (23.5) | 3 (23) | 5 (23.8) | NS |
| Maximal thrombus area, mm2 | 2.5 ± 1.6 | 2.6 ± 1.6 | 2.3 ± 1.6 | NS |
| Maximal malapposition area, mm2 | 0.9 ± 0.8 | 1.1 ± 1.1 | 0.8 ± 0.8 | NS |
| Maximal malapposition length, mm | 2.9 ± 1.7 | 3.7 ± 0.7 | 2.6 ± 2 | NS |
| Plaque rupture adjacent to stent | 10 (29.4) | 2 (15.4) | 8 (38.1) | NS |
| After the intervention | ||||
| Minimal stent area, mm2 | 6 ± 2.7 | 5.8 ± 2.1 | 6.2 ± 3.1 | NS |
| Stent expansion index < 80% | 5 (14.7) | 2 (15.4) | 3 (14.2) | NS |
| Maximal thrombus area, mm2 | 1.4 ± 1.5 | 1.7 ± 1.4 | 1 ± 1.6 | NS |
| Maximal malapposition area, mm2 | 0.5 ± 0.6 | 0.4 ± 0.3 | 0.5 ± 0.8 | NS |
| Maximal malapposition length, mm | 1.4 ± 1.7 | 1.6 ± 1.5 | 1.3 ± 1.9 | NS |
NS, not significant; ST, stent thrombosis.
Unless otherwise indicated, the data represent No. (%) or mean ± standard deviation.
Thrombus burden significantly decreased after treatment (maximal thrombus area, 2.5 ± 1.6mm2 before treatment vs 1.4 ± 1.5mm2 after; P < .001). These outcomes were seen in both patients with early ST (2.6 ± 1.6mm2 vs 1.7 ± 1.4mm2; P = .006) and those with late ST (2.3 ± 1.6mm2 vs 1.0 ± 1.6mm2; P < .001).
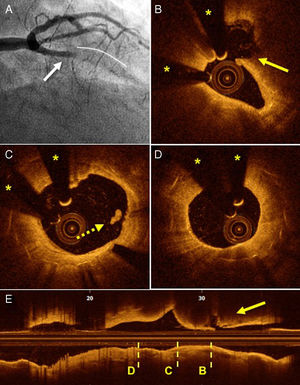
In 8 patients (25%), plaque rupture was seen adjacent to the stent. In all patients, the angiographic imaging was that of a classic ST that met the criteria of the Academic Research Consortium. All of these patients had late ST (4 BMSs and 4 DESs), generally very late (5 patients). In all, the plaque rupture was seen in the coronary segment proximal to the stent and, in 4 of these, was found > 5mm from the proximal edge of the stent. In most of these patients, there were no signs of stent complications, although alterations to the proximal edge could not be completely ruled out in 2 patients due to the presence of thrombotic material with dorsal shadowing (Figure 2).
Angiography and OCT of a patient with late ST secondary to plaque rupture adjacent to the stent. A: thrombotic occlusion of the anterior descending artery (arrow) proximal to the stent (continuous line). B: image of plaque rupture (arrow) adjacent to the stent. C: proximal edge of a completely covered stent, with images of a small thrombus (dotted arrow). D: nonocclusive neoatherosclerosis without indicators of intimal rupture. E: longitudinal cross-section showing the transversal sections corresponding to those of letters B to D. OCT, optical coherence tomography; ST, stent thrombosis. Asterisks indicate shadowing caused by a wire artifact..
No complications were detected that could be directly or indirectly attributed to the use of OCT. Overall in-hospital mortality was 15% (n = 6), 13% (n = 5) of cardiovascular origin, without differences according to ST time.
Early ThrombosisOf the 16 patients who experienced early ST, 5 had a single BMS and 11 had a single DES (Table 1). There were no significant differences in clinical or angiographic variables between the 2 subgroups (Table 1). Optical coherence tomography images of sufficient quality were obtained from 13 of these patients (81%) (Table 2). The most frequently observed findings were malapposition, severe underexpansion, and edge dissection (Figure 3).
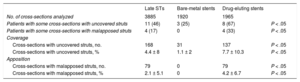
Late ThrombosisOf the 24 patients who experienced late ST, 12 had a single BMS and 12 had a single DES (Table 1). There were no differences in clinical or angiographic variables between late and very late thromboses. Optical coherence tomography images of sufficient quality for analysis were obtained from 21 of these patients (88%) (Table 2). Strut coverage and malapposition data according to stent type are shown in Table 3. Most patients with late ST in a DES showed at least 1 section with uncovered struts while a third of patients had signs of malapposition. In contrast, in late STs in BMSs, only 3 patients had sections with uncovered struts while no patients had signs of malapposition. In addition, image analysis revealed that the group of patients with a DES had a significantly higher percentage of uncovered and malapposed struts. Of the 11 patients with late STs lacking strut coverage, 6 of the STs occurred more than 7 years after implantation.
Strut Coverage and Malapposition Values Studied by Optical Coherence Tomography in Late Stent Thromboses
| Late STs | Bare-metal stents | Drug-eluting stents | ||
|---|---|---|---|---|
| No. of cross-sections analyzed | 3885 | 1920 | 1965 | |
| Patients with some cross-sections with uncovered struts | 11 (46) | 3 (25) | 8 (67) | P < .05 |
| Patients with some cross-sections with malapposed struts | 4 (17) | 0 | 4 (33) | P < .05 |
| Coverage | ||||
| Cross-sections with uncovered struts, no. | 168 | 31 | 137 | P < .05 |
| Cross-sections with uncovered struts, % | 4.4 ± 8 | 1.1 ± 2 | 7.7 ± 10.3 | P < .05 |
| Apposition | ||||
| Cross-sections with malapposed struts, no. | 79 | 0 | 79 | P < .05 |
| Cross-sections with malapposed struts, % | 2.1 ± 5.1 | 0 | 4.2 ± 6.7 | P < .05 |
ST, stent thrombosis.
Unless otherwise indicated, data are expressed as No. (%) or mean ± standard deviation.
Finally, most patients with late ST showed neoatherosclerosis (n = 14; 67%). No differences were seen in the presence of this phenomenon according to stent type (BMS vs DES). Notably, neoatherosclerosis was found to be associated with the region with the highest amount of thrombosis in 11 of the patients. In addition, in most of the patients with neoatherosclerosis (n = 9), the time elapsed since implantation was > 5 years. Stent calcification was frequent (50% of patients). Finally, patients with neoatherosclerosis showed a higher percentage of intimal rupture (93% vs 71%).
DISCUSSIONThe incidence of ST has decreased in recent years due to improvements in newer-generation stents and advances in concomitant antiplatelet therapy.5 However, ST continues to be a gravely serious condition because abrupt thrombotic occlusion of a vessel is associated with extensive infarctions and high mortality.4 Consequently, understanding of the pathophysiological mechanisms underlying this process is vital, with OCT able to provide unique information on the underlying substrate.
The main results of our study are the following: a) use of OCT during ST is safe and feasible and provides singular data on the substrate that cannot be obtained using conventional angiography; b) OCT can guide reinterventions, decrease malapposition length, and visualize the decrease in thrombus burden; c) in a not insignificant number of patients, images were obtained of plaque rupture adjacent to the stent; d) in early STs, the main finding was stent malapposition; e) in late STs, there was a high rate of uncovered and malapposed stents, findings that were highly prevalent in patients with a DES; and f) neoatherosclerosis was very frequent in patients with very late ST.
Optical coherence tomography has higher resolution than other intracoronary imaging techniques but has 2 clear limitations: lower imaging depth and the presence of shadowing behind thrombus images.13 Given these limitations, the use of OCT in clinical situations with a very large thrombus burden, such as ST, can be less useful.
Nonetheless, our results show that OCT is a safe and feasible technique in this challenging clinical setting. Due to its high resolution, despite the presence of residual thrombus in 85% of patients, an adequate analysis of the underlying anatomy was possible. The information provided greatly helped to reach a definitive diagnosis of the possible mechanisms involved.
In addition, systematic OCT study after interventional cardiology helped to guide the technique and optimize the final results. Despite the relatively small size of the present series, this is, to our knowledge, the largest series involving the systematic analysis of OCT results after an intervention. Our findings indicate a very slight increase in the minimal stent area, a decrease in the SEI, a reduction in the maximal malapposition area and length, and a marked reduction in thrombus burden. Some previous studies—with fewer patients but using more aggressive OCT-guided optimization protocols—showed greater changes in the stent after the intervention.4 These differences might be due to the lack of strict optimization criteria and the strong association between underexpansion and highly severe vessel calcification. New studies are required to confirm if more aggressive optimization of stents that develop ST could improve the long-term clinical outcomes of these patients.
In 25% of our patients, plaque rupture was seen adjacent to stents with good coverage that had no evident mechanical problems in their interior. Although this phenomenon has already been reported, the previously reported cases were merely anecdotal.16 The current angiographic criteria for the diagnosis of ST12 are insufficient to detect this underlying phenomenon, which is why we believe OCT to be an indispensable tool for this diagnosis. In addition, the identification of this etiology can have important implications for the treatment of patients with ST. Thus, in these patients, management should be limited to treatment of the plaque rupture, with no need for a new intervention of the previously implanted stent.
By classifying the STs according to time, different pathophysiological mechanisms were identified, as already reported.17–19 The most frequent finding in patients with early ST was stent malapposition, for both acute and subacute STs. Kim et al.18 found a high rate of malapposed struts in asymptomatic patients, as well as a higher prevalence of thrombi associated with these regions of malapposition. There are no solid data showing irrefutable evidence that malapposition after stent implantation is associated with a higher rate of ST. Neither has it been shown that the use of aggressive postdilatation maneuvers to correct malapposition during the initial stent placement reduces adverse events during clinical follow-up.
Almost half of the patients with a late ST had uncovered struts, whereas 17% of them had malapposed struts. Previous autopsy studies found that the most frequent substrate in late ST is delayed or incomplete strut endothelialization.20 Studies21,22 in patients with late ST determined that a mean 12% to 14% of struts were uncovered and that 4% to 6% were malapposed. In our series, the percentages of images showing uncovered or malapposed struts were slightly lower at 4.4% and 2.1%, respectively. This difference could be due to the later development of ST in our series than in the studies mentioned. Although the proportion of uncovered struts decreases over time, these data indicate that this phenomenon can be associated with the onset of ST even several years after stent implantation. A coverage deficiency or malapposition were more common with DESs. Some studies have already reported these findings.14,15 In our sample, this association could be due to the shorter time from stent placement to ST for DESs than for BMSs (median, 740 days vs 3321 days; P < .05). In addition, late occurrence of malapposed struts (acquired malapposition) has been linked to positive vessel remodeling. Because our series lacked OCT data from after the implantation, we could not distinguish between persistent malapposition and acquired malapposition.
Finally, a high percentage of patients with late ST showed neoatherosclerosis. Previous studies have already reported that neoatherosclerosis might be a cause of late ST. In the study by Taniwaki et al.,23 which performed OCT in 64 patients with late DES thrombosis, neoatherosclerosis was detected in 1 of every 4 patients. Again, the higher prevalence of neoatherosclerosis in our study could be due to the longer time elapsed between stent placement and ST.
LimitationsThis study has several important limitations. First, there was no control group. Second, OCT could not be performed in some patients or the image quality was insufficient, which might have led to a selection bias. Currently, OCT does not permit confirmation of correct strut endothelialization because it cannot identify the type of tissue covering the struts. Thus, the term “strut coverage” was preferred in this study. A limitation inherent to the technique is the shadowing effect caused by red thrombi, which causes an unavoidable loss of anatomical information. In addition, evaluation of the degree of strut coverage can be particularly difficult in the presence of thrombi and a laminar thrombus can be indistinguishable from neointimal coverage. The results of the study may be limited by the inability to detect uncovered struts or those with incomplete apposition in regions with considerable thrombotic material. Nonetheless, most patients could be analyzed or provided sufficient information to permit diagnosis. Finally, the lack of a baseline OCT study meant that we could not distinguish between persistent and acquired malapposition.
CONCLUSIONSFor patients who experience ST, OCT is a safe and feasible technique that provides crucial information to establish an accurate diagnosis and guide treatment. Rupture of plaques adjacent to the stent is not infrequent, although, in angiography, it is indistinguishable from a ST originating in the stent. In early STs, the most frequently associated mechanism is malapposition. In late STs, uncovered and malapposed struts and neoatherosclerosis are often seen, particularly in DESs.
FUNDINGThis study was partially funded by the 7th European Framework Program PRESTIGE (PREvention of late Stent Thrombosis by an interdisciplinary Global European effort) (project number: 260309).
CONFLICTS OF INTERESTNone declared.
- –
Stent thrombosis is a serious complication in interventional cardiology. Intracoronary imaging techniques provide crucial data for diagnosis and intervention guidance. Few data are available on the use of OCT for this condition.
- –
Optical coherence tomography of ST is a safe and feasible technique that provides singular information on the underlying pathophysiological mechanisms and can help to guide interventions.