Brugada Syndrome (BrS) is characterized by an elevated ST segment in the right precordial leads (V1-3) on the electrocardiogram (ECG) and an increased risk of ventricular tachycardia/ventricular fibrillation (VT/VF) episodes and sudden cardiac death.1,2 Although more than 2 decades have passed since BrS was first reported by the Brugada brothers,1 few therapeutic options have been found, despite the strong interest in this syndrome. Indeed, at present, there are just 2 therapeutic strategies, which include implantable cardioverter-defibrillator (ICD) and/or chronic quinidine therapy.2 However, quinidine is not effective in many patients and its use is frequently associated with intolerable adverse effects. ICD implantation may be effective in preventing sudden cardiac deaths, and is currently recommended as a class I indication for symptomatic patients with type 1 Brugada ECG pattern who present with aborted sudden death and VF-related symptoms such as syncope, seizure, or nocturnal agonal respiration. Unfortunately, ICD therapy in many patients is associated with inappropriate shocks, lead fractures/failure, and device infections. Additionally, high-risk patients with BrS have recurrent VF episodes, which cause frequent ICD discharges or storms. Rarely, heart transplantation is necessary to control electrical storm.
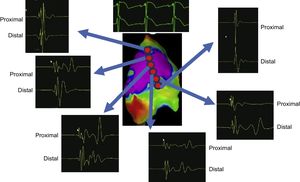
THE DISCOVERY AND CHARACTERIZATION OF THE ARRHYTHMIC SUBSTRATE IN BRUGADA SYNDROMEPast mapping and ablation techniques, initially performed in a limited number of selected BrS patients, targeted both epicardium and endocardium with unsatisfactory results. Currently, there is consensus that potentially arrhythmic substrates responsible for the abnormalities seen in the typical BrS ECG pattern are located on the anterior right ventricular outflow tract of the pericardium.3–10 Lacking effective and well-defined substrate-based therapeutic strategies to control and prevent malignant ventricular tachyarrhythmias in BrS, we first attempted to identify any potential arrhythmic substrates among a large BrS population with different clinical presentations and different baseline BrS ECG patterns.4,6 We did so using combined endo- and epicardial mapping using 3-dimensional potential duration maps before and after ajmaline infusion (1mg/kg in 5minutes). Extensive electrophysiologically well-defined abnormal areas, as unmasked by ajmaline, were accurately identified exclusively on the anterior right ventricular outflow tract and/or right ventricular anterior free wall of the pericardium6 (Figure 1). Wider abnormal areas were found in patients with the worst clinical presentation and/or type 1 BrS ECG pattern. However, in less symptomatic patients, similar large abnormal areas were unmasked after ajmaline infusion.6 In less symptomatic patients without typical BrS-related symptoms, the substrate increased more than 3 times after ajmaline, suggesting that in such a patient population the presence or persistence of modulating or aggravating factors is required to activate the substrate in order to facilitate the development of VT/VF. Overall, the ajmaline-induced substrate increase was larger in men than in women,6 confirming previous studies on a sex difference in BrS. Of interest, low-voltage (< 1mV) fragmented very prolonged potentials were found exclusively in patients with the worst clinical presentation, while in less symptomatic BrS patients we found abnormal ventricular potentials with preserved voltage amplitude.6 While demonstrating the role of extensive complex epicardial substrates, iur substrate-based strategy clearly explains the ineffectiveness of endocardial ablation alone, as well as the lower success of less extensive epicardial ablation not systematically guided by Class Ic drug administration, as initially proposed by others. In our experience, ajmaline rechallenge after ablation can reveal additional abnormal potentials in many patients (> 60%), requiring further RF applications to persistently normalize the ECG pattern.6 Only after ablation of all residual substrates, as confirmed by ajmaline rechallenge, can we persistently normalize the ECG pattern rendering VT/VF noninducible6 (Figure 2). These data and the role of ajmaline infusion are indeed important for successful electrical substrate ablation in BrS. As a result, for the first time, substrate-based ablation strategy, as proposed by our group, could now be available for the vast majority of patients with BrS.
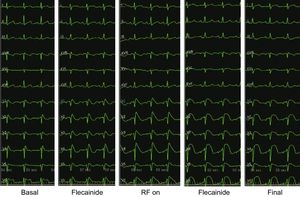
Five consecutive ECGs showing progression of the ST-segment elevation, from basal, after ajmaline, during ablation, after ajmaline again and the final ECG. Note that precordial leads are presented as V1-V2 in second (labeled V1-V2), V1-V2 in third (labeled V3-V4) and V1-V2 in the fourth intercostal spaces (labeled V5-V6) in each ECG. Note the increase in ST-elevation during radiofrequency (RF) delivery and how after ablation ST becomes rounded and is no longer coved-type.
We proposed for the first time ajmaline infusion as a novel method to accurately determine the location and size of arrhythmic electrical substrate (AES) in a series of 14 patients.4 More recently, this strategy, in combination with 3-dimensional duration maps, has been confirmed in a series of an additional 135 patients, proving to be more reliable in identifying patients at risk of VT/VF than risk stratification based on clinical presentation alone, including spontaneous ECG pattern, symptoms, or genetic or family history.6 Ajmaline infusion revealed a significant increase in BrS substrate size, even though most patients with the worst clinical presentation did not have a baseline spontaneous type 1 BrS ECG pattern, a family history of SCD at age < 45 years, or a positive test for SCN5A.6 In our experience, the ability to accurately identify any potential substrate represents the only way to successfully perform RF ablation in order to ensure that the entire AES area is ablated and to minimize the amount of healthy tissue ablated. In 135 consecutive BrS patients, the largest sample size in the world, ablation of the substrate using this method normalized the ECG pattern and made the patients noninducible for VT/VF6 (Figure 3). Epicardial mapping is important for both identifying and ablating the abnormal electrical area. The use of ajmaline together with epicardial mapping is necessary to identify and ablate the AES that can be provoked during certain events, as discussed above. Thus, ajmaline can be particularly useful in patients previously thought to be more “low-risk”, who in reality could suffer VT/VF if provoked by one of many modulating or triggering factors.
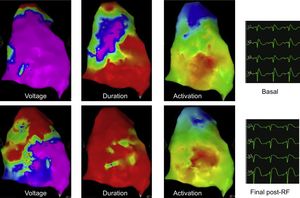
Voltage, duration, and activation maps before and after ablation of the electrical epicardial substrate. ECGs are presented as V1-V2 in the second intercostals space, and V1-V2 in the third intercostal spaces (labeled V3-V4). The voltage map is normal before ablation and slightly decreased in the ablated area. The duration map completely normalized after ablation. Despite the presence of abnormal signals before ablation (the purple area represents signals above 200 msec in duration), no abnormal signals remain after ablation. The activation map shows normal activation sequence and duration before and after ablation, showing that there are no areas of abnormal conduction before the ablation and that the ablation was not transmural because activation remained within the normal limits after epicardial ablation. The ECG shows a clear coved-type ST-segment elevation before ablation and a round-type ST-segment elevation immediately after ablation.
Ajmaline was systematically repeated during follow-up. During a median follow-up of 10 months among 135 symptomatic BrS patients, only 2 (1.5%) with BrS-related symptoms and multiple recurrent VT/VF episodes before ablation experienced just 1 episode of VT/VF after the procedure and in 1 of them electrolyte imbalance was the triggering mechanism.6 These findings are clinically important, demonstrating for the first time an effective therapeutic role of epicardial ablation in preventing VF in a large series of high-risk BrS patients with recurrent VT/VF.
RELATION BETWEEN TYPICAL ECG BRUGADA SYNDROME PATTERN AND SUBSTRATE EXTENSIONWe first demonstrated that regardless of clinical presentation, there is a correlation between the degree of coved-type ST-elevation and substrate magnitude,6 which provides evidence that increases of coved-type ST-elevation in the right precordial leads V1-V3 really represent the corresponding electrophysiological changes of large abnormal right ventricular epicardial substrates. Additionally, normalization of ECG pattern coincided with complete substrate elimination, frequently requiring repeat RF applications.6 These findings clearly suggest that regardless of clinical presentation and/or spontaneous ECG pattern, the BrS population has a well-defined potentially large epicardial substrate, which in many patients may be difficult to activate in the absence of modulating factors or triggers. Our observations explain why a) the development of VT/VF in BrS is a rare and unpredictable event; b) why VT/VF in BrS patients are difficult to manage, frequently requiring several electrical cardioversions for termination; and, c) why electrophysiological study alone is not a good predictor of outcome, particularly in less symptomatic or asymptomatic patients, frequently requiring multiple extrastimuli from different endocardial sites.
COMPLICATIONSNo procedure-related complications occurred in any of our patients and no ventricular tachyarrhythmia developed during ajmaline administration.4,6 Four months after the procedure, only a minority of patients (3%) developed a self-limiting pericardial effusion without acute hemodynamic compromise as detected by echocardiography. They received outpatient care with steroidal or nonsteroidal anti-inflammatory drugs without the use of pericardiocentesis.
CLINICAL IMPLICATIONSThe finding that extensive well-defined epicardial substrates are consistently present in patients with BrS regardless of clinical presentation and/or spontaneous BrS ECG pattern has important clinical implications. Electrophysiological-guided epicardial ablation of the arrhythmogenic substrate can be safely and effectively achieved in an increasing number of high-volume centers, thus reducing the number of events in the high-risk population, potentially limiting the number of ICDs to prevent VT/VF.10
CONCLUSION AND FUTURE DIRECTIONSOur experience in a large cohort of consecutive BrS patients with various clinical presentations, who represent the vast majority of patients currently diagnosed with BrS, has provided new information and insights into understanding the pathophysiology, mechanism, and management of the disease to prevent VT/VF. We conclude that electrophysiologically well-defined extensive abnormal epicardial areas, as determined by ajmaline administration, are the primary site for BrS substrate and are responsible for type 1 BrS ECG pattern and VT/VF inducibility. Persistent ECG pattern normalization without VT/VF inducibility even after repeat ajmaline challenge, indicates that substrate ablation can be considered a safe and potential way of preventing ventricular arrhythmia recurrences and sudden death in most patients with BrS. Based on our large experience, epicardial substrate ablation in patients with BrS is an effective additional therapeutic strategy. We hope that more patients with BrS can be successfully managed by catheter ablation without ICD. However, a randomized study is required to confirm our results. Although no major complications occurred in our patients, these results cannot automatically extend to other less experienced centers worldwide. For high-risk BrS patients with recurrent VF episodes, epicardial ablation may be considered as the treatment of choice, but the benefit of this novel strategy should be balanced with the risks of the ablation procedure.
CONFLICTS OF INTERESTNone declared.