New drug-eluting stents (DES) designed to overcome the limitations of existing devices should initially be tested in preclinical studies. Our objective was to analyze the safety and efficacy of new biodegradable polymer-based DES compared with bare-metal stents (BMS) and commercially available DES in a model of normal porcine coronary arteries.
MethodsWe randomly implanted 101 stents (BMS and biodegradable polymer-based sirolimus-eluting stents: 3 test stent iterations [BD1, BD2, and BD3], Orsiro, Biomime and Biomatrix) in the coronary arteries of 34 domestic pigs. Angiographic and histomorphometric studies were conducted 1 month (n = 83) and 3 months (n = 18) later.
ResultsThe stents were implanted at a stent/artery ratio of 1.31 ± 0.21, with no significant differences between groups. At 1 month, the new test stents (BD1, BD2 and BD3) showed less late loss and angiographic restenosis, as well as lower histologic restenosis and neointimal area (P < .0005), than the BMS. There were no differences in endothelialization, vascular injury, or inflammation between the new test stents and BMS, although the new stents showed higher fibrin deposition (P = .0006). At 3 months, all these differences disappeared, except for a lower neointimal area with the new BD1 stent (P = .027). No differences at any time point were observed between the new test stents and commercially available controls.
ConclusionsIn this preclinical model, the new biodegradable polymer-based DES studied showed less restenosis than BMS and no significant differences in safety or efficacy vs commercially available DES.
Keywords
Drug-eluting stents (DES) have consistently demonstrated lower rates of revascularization than bare-metal stents (BMS) in a wide range of clinical situations1 and thus their use has become widespread, far surpassing that of BMS in Spain.2 The risk of late thrombosis of first-generation DES has been a serious cause of concern.3 This phenomenon has been associated with the deleterious effect of the drug, the drug delivery polymer, the stent platform, or a combination of these in the vessel wall, which leads to incomplete endothelialization and persistent hypersensitivity and inflammatory reactions.4–8
New-generation DES have demonstrated much lower rates of late thrombosis,9,10 which is probably associated with the use of improved polymers. The use of biodegradable polymers has demonstrated an excellent safety profile in preclinical studies12 and clinical studies.13–15 Although these advances are very important, unwanted phenomena persist, such as the appearance of neosclerosis,16 which has prompted the development of new devices that eliminate all such problems.
Preclinical models have demonstrated usefulness in analyzing differences between new devices because the sequence of biological events associated with arterial repair is similar to that in humans.17 The porcine model of healthy coronary artery has been recommended by consensus to assess biological responses after the use of coronary devices.18–21
The aim of this study was to assess the safety and efficacy of 3 new biodegradable polymer-based sirolimus-eluting stent designs in a preclinical porcine model.
METHODSAnimal ModelThis randomized controlled experimental trial with a final blind analysis used 34 Large White domestic pigs aged 2 to 3 months old. All procedures were conducted in accordance with Spanish legal regulations (Royal Decree 53/2013, 1 February, on basic standards for the protection of experimental animals) and European Directive 2010/63EC. The study was approved by the local ethics committee prior to its inception. The randomization method involved the stratified allocation of major coronary arteries so that each type of stent was implanted in the same number of arteries. The predetermined follow-up points were at 1 month (n = 28) and 3 months (n = 6).
Aspirin (325mg) and clopidogrel (300mg) were administered 24hours before the procedure. The anesthesia protocol and surgical preparation have been previously described. 19 The animals received anticoagulant therapy with 5000 IU of unfractionated heparin. To implant the devices at a stent/artery ratio > 1.2, we selected the best location out of 3 epicardial coronary arteries after the administration of intracoronary nitroglycerin.
Devices AnalyzedIn this study, the following devices were used:
- •
Control BMS (n = 27): Conventional bare-metal stent (iVascular), L605 chromium-cobalt alloy, strut thickness 80μm. The stent is constructed of 8 crowns joined by 3 rows of nonconcatenated connectors that form a discontinuous sinusoidal structure to provide better drug distribution.
- •
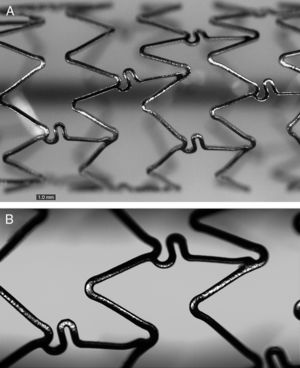
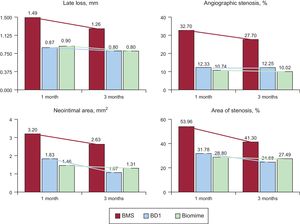
SFA1, BD1 (n = 32): Based on BMS, coated abluminally with 4 to 5μm biodegradable poly(D,L)-lactic-co-glycolic polymer, loaded with 1.0μg/mm2 of sirolimus, with a delivery system that allows more than 60% of the drug to be released within 30 days (Figure 1).
- •
SFA2, BD2 (n = 6): A variant of SFA1 with 1.4μg/mm2 sirolimus.
- •
SFA3, BD3 (n = 3): A variant of SFA1 with a slower release profile, with more than 40% of the drug released within 30 days.
- •
SFA4 (n = 5): Commercially available DES with Biomatrix biodegradable polymer (Biosensors Interventional Technologies, Singapor), strut thickness 120μm, loaded with 15.6μg/mm biolimus in an abluminal matrix of 10 m degradable poly(D, L)-lactate.
- •
SFA5 (n = 16): Commercially available DES with Biomime biodegradable polymer (Meril Life Sciences, India), strut thickness 65μm, loaded with 1.25μg/mm2 sirolimus, with a circumferential degradable matrix of 2μm of poly(L)-lactate and poly(L)-glycolate.
- •
SFA6 (n = 8): Commercially available DES with Orsiro biodegradable polymer (Biotronik, Germany), strut thickness 61μm, loaded with 1.4μg/mm2 sirolimus, with a circumferential degradable matrix of 7.5μm of poly(L)-lactate.
All materials were provided by iVascular, including DES 1, DES 2, and DES 3, which are still not commercially available. The study was designed in conjunction with iVascular, whereas the analysis and interpretation of the results were conducted independently of the company. The sample size and number of stents included in the study were calculated according to consensus documents on preclinical stent analysis.20
Angiographic AnalysisAfter the stents were implanted, coronary angiography was repeated to determine intrastent minimal luminal diameter (MLD). At 1 month (in all animals, n = 34) and at 3 months (in those scheduled for follow-up at this point, n = 6), a new follow-up coronary angiography was conducted to assess MLD during follow-up. The MLD and reference diameters (mean diameter of the segments located in the proximal and distal 5mm of the stent) were calculated using the Medis QCA-CMS automated quantitative coronary angiography analysis software package, version 6.1. We calculated the following angiographic restenosis parameters:
- •
Late loss = initial MLD – follow-up MLD.
- •
Percentage of angiographic restenosis = (1 – [initial MLD/reference diameter]) × 100.
After completing follow-up, the animals were killed (n = 28 at 1 month; n = 6 at 3 months) and histological analysis was performed as previously described.19 The arteries were analyzed histomorphometrically using an Olympus PRovis AX70W digital microscope (Tokyo, Japan) paired with Nikon DXM 1200W digital camera and the ImageJ-NIH Image 1 POINT 4 software package (National Institute of Health, United States). Planimetry was used to determine the luminal area and the internal elastic membrane area and thus calculate the restenosis variables defined by histology:
- •
Neointimal area = internal elastic area – luminal area.
- •
Percentage of stenosis by histology = (1 – [luminal area/internal elastic area]) × 100.
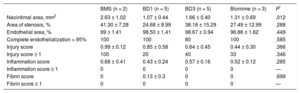
In terms of safety, the histological analysis was based on semiquantitive analysis of 4 parameters18 (Table 1): the degree of vascular injury (injury score), inflammation intensity, persistent fibrin deposition, and the degree of re-endothelization calculated as the approximate percentage of luminal surface covered by endothelial cells. According to the amount of surface area covered by endothelial cells 1 month after implantation, an additional parameter was defined: complete endothelialization, which was at least 95% of the luminal surface covered by these cells.8 Stents with an injury score > 2 were excluded from the final analysis because they can have nonspecific responses in the histology of the arterial wall.
Histologic Injury Score for Safety
| Grade 0 | Grade 1 | Grade 2 | Grade 3 | |
|---|---|---|---|---|
| Injury score | No injury to IEL or media | IEL lacerated; media compressed | IEL and media lacerated; EEL intact | Break in all layers; adventitia involved |
| Degree of inflammation | Absent | Light lymphohistiocytic infiltrate surrounding the stent | Noncircumferential, at least moderate to dense cellular aggregate around the stent | Circumferential, dense cellular aggregate around the stent |
| Fibrin deposition | Absent | Moderate deposition around the stent involving < 1/4 of the artery circumference | Moderate deposition around the stent involving > 1/4 of the artery circumference or heavy deposition around and between the stents involving < 1/4 of the artery circumference | Heavy deposition involving > 1/4 artery circumference |
| Degree of re-endothelialization | Approximate percentage of luminal area covered by endothelial cells Complete endothelialization: at least 95% of the luminal area covered by endothelial cells | |||
EEL, external elastic lamina; IEL, internal elastic lamina.
Published with the permission of Pérez de Prado et al.18
Values are expressed as percentages and as mean ± standard deviation, depending on the type of variable. Semiquantitative variables, such as the safety scores in histopathological analysis, are expressed as mean ± standard deviation (the most common form in previous publications) and as percentages (recommended by consensus documents for preclinical stent studies20).
Differences between the mean of the groups were analyzed using the Student t test and analysis of the variance. For multiple comparisons, a post hoc analysis was conducted using the Dunnett method for comparison with the control BMS and using the Tukey method for comparison of all groups. Semiquantitative variables were analyzed using the chi-square test or the Fisher exact method. To assess the possible influence of different variables (stent/artery ratio, treated artery, stent type, and injury score) on the final results of angiographic and histological stenosis, a multivariate linear regression analysis was conducted that included, in addition to the aforementioned variables, the type of stent (BMS or DES). The variables were entered into the model as a block with an input P value of .05 and an output P value of 0.1. All analyses were conducted with the JMP v10 statistical software package (SAS Institute Inc.; Cary, North Carolina, United States) using a P value < .05 as a cutoff for statistical significance.
RESULTSWe implanted 101 of the 102 planned stents (1 BMS was not implanted because the device was not available at the time), overdilatated to a mean stent/artery ratio of 1.31 ± 0.21, with no differences between stents. All animals completed the planned follow-up and the angiographic studies were performed without incident; however, 1 animal experienced cardiac arrest after anesthesia induction. Histological analyses were performed without incident. All treated segments were permeable. Eight stents (2 BMS, 2 BD1, 2 BD2, 1 BD3, and 1 Biomatrix) were excluded from the final analysis because they had an injury score > 2. Angiographic analysis could not be completed in 6 stents because of the presence of overlapping branches in the segment in 1 case (Orsiro), inadequate opacification in 2 cases (BD1 and Biomime), and in the 3 stents of the animal that died.
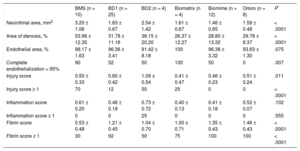
Analysis of Antirestenotic EffectivenessThe angiographic results at 1 month are shown in Table 2. Late loss and stenosis were significantly lower for all the DES than for the control BMS (P < .0001 for both variables). Late loss (P = .0004) and angiographic stenosis (P < .0001) were lower for the new DES (BD1, BD2, and BD3) than for the BMS, but no differences were found vs the commercial DES. At 3 months, angiography was repeated in the 6 scheduled animals and the differences between DES (BD1, BD3, Biomime) and the control BMS disappeared; there was a reduction of stenosis vs the results at 1 month, which was particularly marked for the BMS (–15.3%), but was also evident for the Biomime (–6.7%) and BD1 (–0.6%).
Angiographic Results at 1 Month
| BMS (n = 12) | BD1 (n = 29) | BD2 (n = 4) | BD3 (n = 6) | Biomatrix (n = 4) | Biomime (n = 14) | Orsiro (n = 7) | P* | |
|---|---|---|---|---|---|---|---|---|
| Late loss, mm | 1.49 ± 0.70 | 0.87 ± 0.44 | 0.79 ± 0.35 | 1.00 ± 0.53 | 0.67 ± 0.57 | 0.90 ± 0.34 | 1.04 ± 0.42 | < .0001 |
| Stenosis, % | 32.70 ± 19.04 | 12.33 ± 13.86 | 11.29 ± 11.99 | 18.80 ± 22.65 | 9.35 ± 6.24 | 10.74 ± 10.26 | 4.20 ± 5.45 | < .0001 |
BMS, bare-metal stent; DES, drug-eluting stent.
Data are expressed as mean ± standard deviation.
The histopathological results at 1 month are shown in Table 3 and Figure 2. Neointimal area and stenosis were lower with DES than with BMS (P < .0001 for both variables). Neointimal area (P = .0004) and area of stenosis (P < .0001) were lower with the new DES than with BMS, but no differences were found vs commercial DES. Histological restenosis was lower at 3 months (Figure 3 and Table 4) than at 1-month follow-up. Neointimal area was lower with DES than with BMS (P = .012), especially between BD1 and BMS (1.07 ± 0.44mm2 vs 2.63 ± 1.02mm2; P = .027). However, values were worse with BD3 than with BD1 (1.07 ± 0.44mm2 vs 1.66 ± 0.40mm2; P = .059). The results were similar regarding stenosis (41.30 ± 7.28% vs 24.68 ± 8.99%; P = .070).
Histological Results at 1 Month
| BMS (n = 10) | BD1 (n = 25) | BD2 (n = 4) | Biomatrix (n = 4) | Biomime (n = 12) | Orsiro (n = 8) | P* | |
|---|---|---|---|---|---|---|---|
| Neointimal area, mm2 | 3.20 ± 1.06 | 1.83 ± 0.67 | 2.54 ± 1.42 | 1.61 ± 0.67 | 1.46 ± 0.65 | 1.59 ± 0.48 | < .0001 |
| Area of stenosis, % | 53.96 ± 12.35 | 31.78 ± 11.16 | 36.15 ± 20.20 | 26.37 ± 12.27 | 28.80 ± 13.32 | 29.78 ± 8.37 | < .0001 |
| Endothelial area, % | 98.17 ± 1.63 | 96.36 ± 3.41 | 91.42 ± 8.18 | 100 | 96.38 ± 3.32 | 93.63 ± 1.30 | .075 |
| Complete endothelialization > 95% | 90 | 52 | 50 | 100 | 50 | 0 | .007 |
| Injury score | 0.93 ± 0.33 | 0.60 ± 0.42 | 1.09 ± 0.54 | 0.41 ± 0.47 | 0.46 ± 0.23 | 0.51 ± 0.24 | .011 |
| Injury score ≥ 1 | 70 | 12 | 50 | 25 | 0 | 0 | < .0001 |
| Inflammation score | 0.61 ± 0.20 | 0.46 ± 0.18 | 0.73 ± 0.72 | 0.40 ± 0.13 | 0.41 ± 0.18 | 0.52 ± 0.07 | .102 |
| Inflammation score ≥ 1 | 0 | 0 | 25 | 0 | 0 | 0 | .555 |
| Fibrin score | 0.53 ± 0.48 | 1.21 ± 0.45 | 1.04 ± 0.70 | 1.00 ± 0.71 | 1.35 ± 0.43 | 1.46 ± 0.43 | < .0001 |
| Fibrin score ≥ 1 | 30 | 92 | 50 | 75 | 100 | 100 | < .0001 |
BMS, bare-metal stent; DES, drug-eluting stent.
Data are expressed as percentage or mean ± standard deviation.
Histological Results at 3 Months
| BMS (n = 2) | BD1 (n = 5) | BD3 (n = 5) | Biomime (n = 3) | P* | |
|---|---|---|---|---|---|
| Neointimal area, mm2 | 2.63 ± 1.02 | 1.07 ± 0.44 | 1.66 ± 0.40 | 1.31 ± 0.69 | .012 |
| Area of stenosis, % | 41.30 ± 7.28 | 24.68 ± 8.99 | 38.18 ± 15.29 | 27.49 ± 12.99 | .288 |
| Endothelial area, % | 99 ± 1.41 | 98.50 ± 1.41 | 98.67 ± 0.94 | 96.86 ± 1.62 | .449 |
| Complete endothelialization > 95% | 100 | 100 | 80 | 100 | .585 |
| Injury score | 0.99 ± 0.12 | 0.85 ± 0.58 | 0.64 ± 0.45 | 0.44 ± 0.30 | .366 |
| Injury score ≥ 1 | 100 | 20 | 40 | 33 | .346 |
| Inflammation score | 0.68 ± 0.41 | 0.43 ± 0.24 | 0.57 ± 0.16 | 0.52 ± 0.12 | .285 |
| Inflammation score ≥ 1 | 0 | 0 | 0 | 0 | — |
| Fibrin score | 0 | 0.13 ± 0.3 | 0 | 0 | .699 |
| Fibrin score ≥ 1 | 0 | 0 | 0 | 0 | — |
BMS, conventional stent; DES, drug-eluting stent.
Data are expressed as percentage or mean ± standard deviation.
We performed multivariate analysis and the model with the greatest predictive power showed an independent association between stenosis and a higher injury score (B = 18.43; 95% confidence interval, 12.02-24.84; P < .0001) and lower stenosis in DES (B = –16.84; 95% confidence interval, −23.55 to −10.13; P < .0001).
Safety AnalysisSafety variables at 1 month are shown in Table 3. Endothelialization was greater with BMS than with DES (P = .075 in the quantitative analysis: P = .007 in the semiquantitative analysis). The results of the injury score were the opposite: there was a lower quantitative value (P = .011) and fewer stents with an injury score ≥ 1 (P < .0001) with DES than with BMS. The degree of inflammation was low for nearly all the stents (less than grade 1). Fibrin deposits were lower in the BMS group than in the tested DES (P < .0001). In the individual analysis of the BD1 stent vs BMS, significant differences were found in favor of the BD1 in the injury score and in the degree of inflammation (P < .036 in both cases).
At 3 months, all differences disappeared (Table 4). Vascular injury and inflammation scores were very similar to those observed at 1 month, with the disappearance of fibrin deposits and almost complete endothelialization.
DISCUSSIONThis study analyzed the safety and efficacy of new biodegradable polymer-based sirolimus-eluting stents compared with BMS and 3 commercially available DES. The preclinical model showed that the new devices decreased the degree of restenosis in a sustained way up to 3 months (particularly BD1) compared with control BMS and with safety parameters equivalent to those of commercially available DES. In certain aspects (inflammation, vascular injury), the new devices were potentially superior to BMS.
Antirestenotic EfficacyThe new DES generally demonstrated greater efficacy in the prevention of restenosis than control BMS. Previous studies have shown that different biodegradable polymer-based sirolimus-eluting DES significantly decreased late loss and revascularization of the treated vessel compared with BMS15 and obtained similar results to those of durable-polymer DES.13–15 Individual analysis showed that BD1 and BD2 stents showed similar and significantly better restenosis values than those of BMS; however, BD3 showed worse values, especially during the 3-month follow-up, and therefore their use was discontinued in the following phases of the study.
The experimental model of healthy coronary arteries was based on the induction of vascular injury by the overdilatation of the stents and, as previously described,22 a greater degree of injury leads to greater neointimal proliferation. Although the stent/artery ratio in this study was higher than the recommended ratio,20 it is noteworthy that it was not associated with an excessive injury score that could induce nonspecific vascular responses. Less than 8% of the stents showed a value ≥ 2, without differences between stents. In view of the possible impact of eliminating these data on the overall results, they were included in subsequent analyzes and no relevant variation was observed. In the multivariate analysis, the use of DES was independently associated with a lower degree of stenosis, confirming that the effect is real and is not exclusively related to arterial injury.
Safety of New StentsThe degree of endothelialization was worse with DES than with control BMS. This response may be explained by the effect of sirolimus, a potent inhibitor of endothelial proliferation via the deactivation of the p70 S6 kinase pathway, which is a fundamental step for cell cycle progression in response to growth factors.23 Differences between BMS and BD1 were lower than with the overall DES group and did not reach statistical significance (P = .121 in the quantitative analysis; P = .055 in the semiquantitative analysis).
Despite the high stent/artery ratio, all the stents analyzed had low injury scores with a mean value of < 1. Drug-eluting stents showed a significantly lower degree of vascular injury than BMS. Although the stent/artery ratio was similar in both groups (1.34 ± 0.19 vs 1.30 ± 0.22; P = .535), we cannot rule out the possibility that this difference was influenced by some uncontrolled variable. However, this finding also reflects the high degree of biocompatibility of the tested materials.
Likewise, DES tended to show a lower inflammation score (P = 0.102) than BMS. In the specific case of BD1 stents, the degree of inflammation and vascular damage were significantly lower (P < .036) than with BMS. This finding may be explained by several factors: the anti-inflammatory effect of the drug, the optimized stent design for better drug release, and the contribution of the biodegradable polymer. The inflammation scores were significantly lower than those observed in a previous study with durable-polymer DES.19 Although it is not possible to make direct comparisons, these findings have already been described in other series.11,12
Fibrin deposition around the stents was greater in the DES group, which indicates the effect of the drug.18,19 As previously mentioned, its disappearance at 3 months is an interesting finding.
In the same way that differences were found between BD1 and BD3, BD2 showed a significantly higher injury score and inflammation scores and worse endothelialization data than BD1. Thus, BD2 was eliminated from the final phases of the study.
Mid-term Outcome (3 months)The histological restenosis parameters showed a reduction of the neointimal area and the area of stenosis compared with that observed at 1 month in all stents. This phenomenon has been previously described,24 although it does not always appear in all DES; in fact, paclitaxel-eluting DES tend to show a greater late loss.25 This phenomenon may be explained by the substitution of type III collagen for type I collagen, which causes retraction of the neointima, as in the healing of any injury, and a reduction in the density of smooth muscle cells.26
The safety variables showed very favorable 3-month outcomes, with no major inflammation or late vascular damage and with complete endothelialization. The disappearance of fibrin could reflect the rapid release of the drug during this period, as well as the biocompatibility of the polymer.
LimitationsLike all preclinical models, this study has intrinsic limitations, because no animal model can reproduce with complete fidelity the complex characteristics of human coronary disease.20 Diseased animal models may reflect some of these characteristics, but their validity remains unverified. Although the activity of the drug and the behavior of the stent can be assessed on the basis of the proliferative response, it is unknown if this effect would be the same in arteries with high atherosclerotic content, in which fragmentation of the internal elastic lamina appears spontaneously as part of the general inflammatory process, particularly in vulnerable plaques, in which other mediators participate in the vascular repair process. Nevertheless, the porcine coronary artery model remains the one recommended by consensus documents for device assessment. Histologic assessment using semiquantitative variables also limits accuracy; however, we followed the standards for analysis recommended by expert consensus.20 The BMS used was a noncommercial variant of the Architect stent with no specific safety/efficacy information and was used as the basic structure of the DES analyzed. The sample size was small, although it was selected following the standards recommended by the aforementioned consensus documents. Although the power to detect differences with the BMS was adequate, the power to detect differences between DES was lower, and so these comparisons must be interpreted with caution.
CONCLUSIONSIn an experimental healthy porcine coronary artery model with the implantation of overdilatated stents, new biodegradable polymer-based sirolimus-eluting stents demonstrated a significant reduction in restenosis compared with BMS and this effect was maintained at 3 months. Their safety profile was similar to that of BMS at 3 months. In addition, no relevant differences were found with the biodegradable polymer DES currently available on the market.
FUNDINGLVD Biotech/Vascular provided all of the study stents and provided financial support for performing the experimental procedures and histologic analyzes.
CONFLICTS OF INTERESTA. Pérez de Prado and F. Fernández Vázquez have been consultants and have received support for this research project and others from LVD Biotech/Vascular. M. Molina Crisol, M. Amorós Aguilar, I. Pérez Serranos, A. Vidal Parreu, A. Benavides Montegordo, and L. Duocastella Codina are employees of LVD Biotech/Vascular. L. Duocastella Codina is CEO (Chief Executive Officer) and owns shares in LVD Biotech/Vascular.
- –
Although new-generation DES solve many of the problems of older DES, there is still room for improvement in aspects such as the development of neoatherosclerosis. Progress in the development of drug delivery polymers could help in this task. Before clinical comparisons can be made, new developments must be tested in preclinical models; the healthy porcine coronary artery model remains that recommended by consensus documents to evaluate efficacy and safety.
- –
The analysis of these new biodegradable polymer-based sirolimus-eluting stents demonstrates their sustained efficacy over time (up to 3 months) in the form of lower restenosis (angiographic and histologic) than that found with conventional stents and similar to that found with other commercially available stents. In terms of vascular injury, inflammation, persistent fibrin deposition, and re-endothelization, their safety is comparable to that of commercially available DES at 1 month and is similar to that of BMS at 3 months.