Stent/scaffold thrombosis (ST) is one of the most catastrophic consequences after percutaneous coronary intervention (PCI). Understanding its causes might help not only in its prevention and treatment but also in the design of newer generations of intravascular devices. ST is categorized into acute ST (AST, occurring ≤ 1 day following PCI), subacute ST (SAST, 1 day to 1 month following PCI), late ST (LST, 1 month to 1 year following PCI) and very late (VLST, > 1 year following PCI).1 In-hospital mortality is not low regardless of timing (7.9% in AST+SAST, 3.8% in LST, 3.6% in VLST).2 Optical coherence tomography (OCT) and optical frequency domain imaging are the highest-resolution intracoronary imaging technologies available to visualize the arterial wall at a micrometer level of resolution, approximately 10 times greater than intravascular ultrasound. OCT or optical frequency domain imaging are probably the best available techniques to assess the causes of ST.
In a recent article published in Revista Española de Cardiología, Cuesta et al.3 reported OCT findings in 40 consecutive patients with AST+SAST (n = 16 patients) and late and LST+VLST (n = 24) from a single center. Stent type varied (17 were bare-metal stents [BMS], 4 were first generation drug-eluting stents [DES], 12 were second generation DES, 4 were fully bioresorbable scaffolds and 3 were unknown stents). Because of a lack of baseline imaging or insufficient image resolution, 6 OCT images were excluded from the analysis and 13 AST+SAST and 21 LST+VLST were assessed. In AST+SAST, which included 5 BMS and 11 DES, the most frequent cause of ST was malapposition (39%, 5/13) followed by underexpansion (23%, 3/13) and edge dissection (23%, 3/13) and 2 had no specific finding. In LST+VLST, which included 12 BMS and 12 DES, most DES (67%) had evidence of some uncovered struts, while this was much less frequent in BMS (25%). About one-third of DES had some evidence of malapposition, while no BMS showed evidence of malapposition. Neoatherosclerosis, defined as the presence of neointimal lipid, calcification or both, was prevalent in 67% of late ST lesions with no differences between BMS and DES. Interestingly, neoatherosclerosis was found to be associated with the region with the highest amount of thrombus burden and in most cases with neoatherosclerosis (9/14); the time elapsed since implant was > 5 years.
In recent years, similar studies have been conducted examining OCT finding in patients with ST.4–8 Souteyrand et al.4 reported OCT findings from 120 ST cases from the PESTO French registry. The major mechanism of AST+SAST was malapposition (48%) followed by severe underexpansion (26%). The mechanisms of LST+VLST were more varied (malapposition [32%], neoatherosclerosis rupture [28%], coronary evagination [10%], uncovered strut [10%]). Taniwaki et al.5 studied the OCT findings of VLST (median duration, 4.7 years) in first-generation DES (n = 38) and second-generation DES (n = 20) from a European registry. Similarly, the most frequent findings were strut malapposition (34.5%), neoatherosclerosis (27.6%), uncovered struts (12.1%), and stent underexpansion (6.9%). More recently, Lee et al.8 reported an OCT study of VLST (median duration, 4.6 years) in first-generation DES (n = 71) and second-generation DES (n = 27) from 10 South Korean hospital registries.8 Major mechanisms of VLST were neoatherosclerosis (34.7%), stent malapposition (33.7%), and uncovered struts without stent malapposition or evaginations (24.5%).
Collectively, these data provide a wealth of knowledge about the causes of ST in living patients. In cases of AST, the study by Cuesta et al., as well as others, suggests that in most cases of AST+SAST, there is evidence of some stent- (eg, underexpansion) or procedural- (eg, malapposition or dissection)-based complication. Indeed, in our own pathologic study, we assessed 37 lesions in 34 patients with early ST in comparison with 25 patients/lesions with patent stents; all of the patients died within 30 days of stent implantation.9 Among the independent predictors of early ST were necrotic core prolapse, medial tear, and incomplete stent apposition. In this series, 34% of sections showed the presence of malapposition vs 18% in those with patent stents. Malapposition distance was also greater in those with thrombus (204μm) vs those without (51μm), suggesting that the degree of malapposition is an important determinant of thrombosis risk. The study by Cuesta et al. does not provide a control group (ie, patients without ST) for comparison, which would have provided interesting data regarding relative differences in the degree of malapposition. We can probably assume that the degree of malapposition was large, given that these cases went on to thrombose. Other studies have confirmed that acute stent malapposition tends to be fairly prevalent in cases of BMS and DES treatment for ST-elevation myocardial infarction, probably because overlying thrombus obscures the arterial wall dimensions.10 Although not provided, it would have been interesting to know the clinical indications for stenting in these patients.
The current study also has important implications for the treatment of early ST. Most of the mechanisms described would not be solved by the additional placement of stents, with the exception of residual flow-limiting dissections. In cases of malapposition, the treatment strategy for ST should be relatively straightforward, as better apposition will likely improve outcomes. Similarly for stent underexpansion, more complete postdilation is needed with evidence of better stent expansion. While it might be expected that imaging at the time of stent implantation would improve outcomes from the standpoint of early ST, in the multicenter randomized controlled ILLUMIEN III trial, using either OCT-guided, intravascular ultrasound-guided, or angiography-guided stent implantation, there was no significant difference between groups in terms of major adverse cardiac events at 30 days.11
Cuesta et al. also provide interesting data on the causes of late ST. The relatively common findings of uncovered struts, especially in the DES group, is probably not surprising given the known effect of DES on delayed healing and our previous finding in pathologic studies that uncovered struts are an important risk factor for ST. As expected, the frequency of uncovered struts was significantly lower in patients receiving BMS. We are also not informed about whether uncovered struts were more frequent in first-generation DES vs second-generation DES, although most DES in this study were second-generation DES. In another pathologic series, we demonstrated that the number of uncovered struts was significantly lower in second-generation cobalt chromium EES vs first-generation DES. Thus one might expect to find uncovered struts less frequently in second-generation DES.12
Of additional interest is the role of malapposition in late ST. Cuesta et al. report no cases of malapposition in BMS vs 33% in DES cases. Whether the cases of DES malapposition were acute and persistent or late-acquired is not shown but one would assume such data is available given these patients had baseline imaging. Moreover, it would have been interesting to understand how many cases of second generation DES had both malapposition and uncovered struts. Foin et al.13 examined the relationship between the degree of malapposition, flow disturbances, and neointimal coverage of stent struts using a retrospective OCT study. Struts with malapposed distance < 100μm had minimal disturbances of blood flow compared with floating struts with more significant malapposition distance. Segments with malapposition < 100μm had a minimal impact on strut coverage, while distances 100 to 300μm and > 300μm had significantly more uncovered struts. Given the significantly better healing of second-generation DES, it might be expected that some additional factors such as malapposition contribute to the delayed healing seen in these cases.
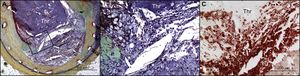
Last, Cuesta et al. also provide important insights into the role of neoatherosclerosis in late ST (Figure). It is known from both pathology and clinical data that neoatherosclerosis progresses more rapidly and frequently in DES than in BMS.15 Our own autopsy analysis of BMS ≤ 2 years of implant duration demonstrated no evidence of neoatherosclerosis, whereas there was a progressive increase in its incidence in stents with implant duration > 2 years but ≤ 6 years (22%), and in those with implant duration > 6 years (42%). The data reported by Cuesta et al. conflict with our own in that they found late ST from neoatherosclerosis equally in BMS and DES with 9 of 14 stents with implant duration > 5 years. Clinicians should be aware that neoatherosclerosis as a cause of late ST in BMS and DES is indeed very likely. The findings of Cuesta et al. differ radically from our neoatherosclerosis studies, in which DES stents with < 2 years of implant duration showed an incidence of 29%, increasing to 44% in DES > 2 years but < 6 years of implant duration. This makes sense given that endothelial dysfunction is greater in DES than in BMS and that more advanced plaque formation is observed less rapidly in native vessels and is significantly more rapid in DES. Ultimately, the development of neoatherosclerosis limits the durability and safety of current-generation DES and further work needs to be conducted to understand its exact causes.
Very late stent thrombosis due to neoatherosclerosis with in-stent plaque rupture. (A) In-stent plaque rupture with luminal occlusive thrombus (Thr) within bare-metal stent without restenosis. A large number of macrophages were identified at the ruptured cap (B) by immunostaining using an anti-CD68 antibody (C). Reproduced with permission from Nakazawa et al.14
In conclusion, the study by Cuesta et al. further illuminates an important subject in interventional cardiology. Outcomes in patients with ST continue to be poor with 15% of patients in the current study dying from their ST event. Understanding the temporal causes of ST will help interventional cardiologists to appropriately treat this devastating event. All interventionists should be aware of the importance of minimizing malapposition, especially when it is large, and ensuring adequate stent expansion in preventing early ST events.16 The causes of very late ST are more complex and likely cannot be solved simply by stent implantation techniques alone. Chief among very late ST causes is neoatherosclerosis, which was found in a large number of cases reported by Cuesta. Greater efforts must be made to understand its mechanisms if we are to improve long-term outcomes in patients receiving stents for the treatment of coronary artery disease.
CONFLICTS OF INTERESTCVPath have received institutional research support from 480 Biomedical, Abbott Vascular, ART, BioSensors International, Biotronik, Boston Scientific, Celonova, Claret Medical, Cook Medical, Cordis, Edwards Lifescience, Medtronic, MicroPort, MicroVention, Celonova, OrbusNeich, ReCore, SINO Medical Technology, Spectranetics, Surmodics, Terumo Corporation, W.L. Gore and Xeltis. H. Mori has received honorarium from Abbott Vascular Japan, Goodman and Terumo Corporation. A.V. Finn has sponsored research agreements with Boston Scientific and Medtronic CardioVascular and is an advisory board member to Medtronic CardioVascular.