Since the development of transcatheter aortic valve implantation (TAVI) and its first-in-man use by Alain Cribier in 2002, TAVI has become the gold standard for symptomatic severe aortic stenosis (AS) in patients considered inoperable or at high risk for perioperative death.1 TAVI has recently been shown to provide similar results to aortic valve replacement in terms of outcomes and quality of life (QoL).
Since 2002, the technology for the TAVI procedure has been refined and the numbers of implantations has increased over the last few years, with more than 500 000 procedures performed in over 70 countries worldwide. TAVI is now a routine intervention and its indications have been expanded from only inoperable and high-risk patients at first to now include those at intermediate risk and even those deemed low risk.2 In a recent viewpoint article on the future of TAVI, Kim and Hamm hypothesized that TAVI might become the first-line therapy for moderate-to-severe aortic valve stenosis (or aortic regurgitation) regardless of symptoms or operative risk 10 to 15 years from now.3
Although TAVI is a routine procedure today, there are still patients who have symptomatic severe AS not benefiting from this costly interventional procedure with its inherent risks, including stroke, bleeding, and death. Current Heart Team consensus is that patients scheduled for TAVI should have reasonable QoL and a minimum life expectancy of at least 1 year. Patients with severe dementia, severe frailty, those who are bedridden, and those with end-stage concomitant diseases (eg, end-stage kidney or liver disease or chronic obstructive pulmonary disease) or metastatic cancers are considered not to benefit from TAVI. Diagnosing and detecting these patients is crucial to avoid unnecessary procedures causing avoidable costs but, most importantly, to avoid harm in terms of complications and hospitalization, therefore reducing QoL.
RISK ASSESSMENT IN TAVIRisk assessment is considered paramount in patient selection for TAVI. Scoring systems, such as the Society of Thoracic Surgeons (STS) and the logistic EuroSCORE, are used, but are only weakly correlate with true peri-interventional risk.4 In addition, frailty (weakness, muscle wasting, malnutrition, and slow gait speed) and neurocognitive function are major factors influencing outcome after TAVI.5 However, the available scoring systems are complex to calculate, and frailty might be difficult to assess. Furthermore, distinct methods to quantify frailty are available. Frailty is usually measured by clinical assessment combining physical examination with information about everyday capabilities. Several scores are available but the Clinical Frailty Scale has been successfully tested against other tools and is relatively easy to use.6 Recently, Gilbert et al.7 recently proposed and validated another approach to quantify frailty by adding up clinical data from ICD-10 codes. This approach could help to objectify frailty and contribute to risk prediction in TAVI patients.
While objective parameters for risk assessment, which are easily measured and at low cost, contribute to the Heart Team decision-making processes, biomarkers might also be ideal candidates. Several biomarkers have already been tested in TAVI cohorts and have proven their prognostic importance and strengths.
GALECTIN-3 AND CA125 IN ONCOLOGY AND HEART FAILUREIn a recent paper published in Revista Española de Cardiología, Rheude et al.8 tested galectin-3 and carbohydrate antigen 125 (CA125) for risk assessment in patients undergoing TAVI. Galectin-3, a member of the lectin family, plays an important role in inflammation, tissue repair, and cardiac fibrosis. Especially in heart failure (HF), galectin-3 has been shown to be a helpful biomarker for prediction of prognosis and risk stratification. Experimental models have determined that galectin-3 is involved in cardiac fibrosis and in pathologic remodelling of the heart.9 Galectin-3 is also mentioned in the guidelines of the American College of Cardiology Foundation/American Heart Association for the management of HF as a prognostic biomarker in HF patients (class IIb indication).10
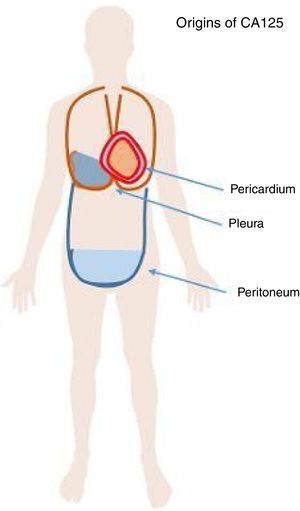
CA125 is a glycoprotein of the mucin family. This glycoprotein is known to be released from the cell surface after proteolytic cleavage and can be detected in blood, pleural and pericardial effusion, and ascites (Figure 1). CA125 was first detected in ovarian cancer cell lines and has been used as a marker for treatment monitoring in ovarian cancer for many decades in the clinics. In other cancers, such as lung cancer, teratoma and non-Hodgkin lymphoma, elevated CA125 levels have also been described in liver cirrhosis. CA125 is also produced by pericardial and pleural tissue and several studies have described elevated CA125 levels in patients with decompensated HF, especially in those presenting with pleural effusion. In patients with HF with preserved ejection faction (HFpEF), CA125 was reported to be elevated. It was hypothesized that CA125 is released due to stress on mesothelial cells as a response to hemodynamic and inflammatory stimuli.11
RISK STRATIFICATION USING BIOMARKERS IN TAVI PATIENTSRisk stratification and patient selection is of paramount importance in patients scheduled for TAVI. Scoring systems and established cardiac biomarkers such as N-terminal probrain natriuretic peptide (NT-proBNP) might help decision-making by the Heart Team but are not sufficient to stratify the risk of an individual patient.
An approach combining distinct tools for risk stratification seems intriguing. The group of Hengstenberg12,13 focussed on this aspect in previous studies and sought to analyze prognostic applicability of scoring systems together with CA125 in the context of AS and TAVI (eg, NT-proBNP and logistic EuroSCORE).
Other novel cardiovascular biomarkers have been the focus of research, such as suppression of tumorigenicity 2 (ST2) or growth differentiation factor 15 (GDF-15), which has been investigated in clinical trials in this context.14,15 ST2 is of particular interest as it was shown to be superior or at least comparable to NT-proBNP in predicting adverse outcomes not only after TAVI but also in HF with both preserved and reduced ejection fraction.16
Another interesting biomarker might be insulin-like growth factor binding protein 2 (IGFBP2), which was shown to outperform elaborate scores like EuroSCORE and STS.17 Other insulin-like growth factors (IGFBP7) were shown to predict diastolic dysfunction; therefore, IGFBP might provide additional insight compared with traditional cardiovascular biomarkers.18
IMPLICATIONS OF ELEVATED CA125 FOR NONONCOLOGICAL PATIENTSCA125 is released by mesothelial cells of the peritoneum, pericardium and pleura due to stress stimuli. CA125 is a marker of inflammation or nonspecific tissue irritation and is not specific for malignant diseases. Therefore, it is not recommended to screen patients showing elevated CA125 without other symptoms of malignant disease. CA125 is not diagnostic for any cancer and is established only for monitoring malignant disease, in particular ovarian cancer. Elevated CA125 levels due to HF could mislead physicians to suspect the presence of cancer, as described by Hopman et al.19 Knowing the exact pathophysiological background of elevated CA125 is therefore essential to avoid misinterpretations of isolated laboratory test results. This issue might gain importance if, based on recent results, CA125 were to be established as a marker protein for cardiovascular disease monitoring in the future.
ECONOMIC IMPLICATIONSParallel to the rise in TAVI numbers, novel biomarkers for risk assessment and for follow-up monitoring have been investigated in manifold studies using elaborate strategies. However, analytics of novel protein-based biomarkers using sandwich enzyme-linked immunosorbent assays (ELISA) done by hand or using automated immunofluorescent assays for quantitative determination, as performed in most clinical trials studying cardiac biomarkers in TAVI patients, require extensive technical and financial resources. This could prove to be impractical, laborious and time-consuming, especially when analyzing large numbers of samples. These factors might hinder the widespread use of novel biomarkers.
These issues can be avoided when using CA125, as this marker is already long established in clinical laboratories and its analysis is implemented in routine testing. Furthermore, the measurement of CA125 levels in patients’ blood samples is considerably cheaper compared with cardiac biomarkers such as NT-proBNP. Ordu et al.20 reported that performing a test for CA125 is 10 times less expensive than conducting an NT-proBNP test (approximately $1 vs $11, data from mid-January 2012).
TECHNICAL ISSUESCA125 is a marker protein that has been used in the clinical setting for many years with standardized testing. However, large-scale laboratory testing for galectin-3, ST2, or IGFBP2 is not common. In most studies investigating novel biomarkers, commercially available ELISA kits are used and their accuracy might not meet the high-precision requirements of routine parameters measured in large, streamlined laboratory testing facilities.
FUTURE RESEARCHAs mentioned by Rheude et al.,8 models for risk prediction in TAVI patients remain inadequate to date. Biomarkers could potentially aid Heart Team decisions and provide further necessary information in everyday clinical settings.
The knowledge of different pathophysiological pathways involved in disease progression in AS might be better depicted using biomarker assessment, which would facilitate earlier diagnosis and better risk assessment. Although challenging, the development of novel biomarker concepts, eg, by means of proteomics/mass spectrometry or next-generation sequencing (such as microRNA analysis) could yield interesting new findings from a basic science point of view. Nevertheless, such analyses are complex, time-consuming and expensive, which may hamper their use in large clinical trials.
Using already known biomarkers, such as CA125, applied in other contexts or disease settings, might obviate this in a cost-efficient and elegant way.
Biomarkers could facilitate decision-making processes, particularly in very high-risk patients. We believe that patients at “extreme” risk could be identified by combining simple clinical information, such as left ventricular ejection fraction, pulmonary hypertension, and also frailty, together with biomarkers: the combination and integration of clinical information, patients’ general capabilities (frailty), and robust markers for risk prediction (biomarkers) could help to predict risk. Potential biomarkers of interest include CA125, galectin-3, ST2, and IGFBP2. An integrated approach might be particularly helpful in deciding between a conservative and an interventional treatment strategy (Figure 2). It could further help in identifying areas of vulnerability: in a patient with good ejection fraction, low pulmonary pressure, and a favorable biomarker constellation but high frailty, clinicians and geriatricians could work together to try to reduce frailty and then re-evaluate the patient. However, further prospective studies are warranted to substantiate this notion.
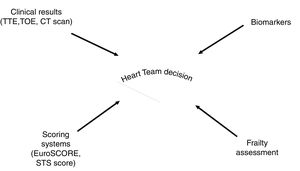
Risk assessment for decision finding in the interdisciplinary Heart Team should be based on clinical results, scores, and frailty assessment. The use of novel biomarkers might be helpful in this process. CT, computed tomography; STS score, Society of Thoracic Surgeons score; TTE, transthoracic echocardiography; TOE, transoesophageal echocardiography.
CA125, previously considered only as a marker for ovarian cancer and its therapeutic monitoring, is attracting more and more attention in HF research, as it is cheap and already established in many laboratories. The combination of CA125 with other novel biomarkers in combination with simple clinical information and frailty assessment could improve the risk stratification of patients evaluated for TAVI.
CONCLUSIONSeveral biomarkers have been shown to add predictive value to established risk scores. CA125, galectin-3, ST2 and IGFBP2 are among the most promising biomarkers for predicting risk in TAVI patients. CA125 might be of particular interest as it is inexpensive and already established in most laboratories. An integrated approach combining potent biomarkers with clinical information and assessment of frailty might be easy to implement and optimize risk stratification in TAVI patients.
CONFLICTS OF INTERESTNone declared.