Scientific advances have led to the discovery of a broad range of novel biomarkers associated with cardiovascular disease progression, which may enhance risk assessment and improve cardiovascular morbidity and mortality independently of previously described traditional risk factors such as hypercholesterolemia and, in the case of heart transplant (HT), donor-specific antibodies (DSA).1 Some of these biomarkers could become important diagnostic tools in clinical practice. For example, ST2 has been suggested as a potential biomarker that has been associated with allograft rejection in HT recipients.2 One such biomarker that has been described to be associated with prognosis in heart failure (HF) is galectin-3 (Gal-3). Gal-3 is a β–galactoside-binding lectin secreted by activated macrophages, which has gained interest as a novel biomarker reflecting inflammation and tissue fibrosis. Elevated Gal-3 levels are associated with left ventricular dysfunction and poor prognosis in patients with HF,3 as well as with an increased risk of incident HF and mortality in the general population.4 However, there is a paucity of data on the longitudinal change in serum Gal-3 levels with improvement in cardiac function, their change with reversal of the HF state after HT, and their prognostic value post-HT.
Myocardial fibrosis has been described in serial endomyocardial biopsies of cardiac allografts and was found to be an important contributor to the development of restrictive cardiac physiology in HT recipients.5 Although Gal-3 has been shown to be associated with fibrosis and remodeling in HF patients, higher serum Gal-3 levels after HT were not associated with advanced replacement myocardial fibrosis or the grade of cardiomyocyte hypertrophy of the cardiac allograft based on evaluation of endomyocardial biopsies at 3 years post-HT.6 Therefore the importance and role of Gal-3 after HT requires further study.
In a recent article published in Revista Española de Cardiología, Suárez-Fuentetaja et al. address this important issue7 by retrospectively analyzing the banked serum samples of 99 participants for Gal-3 levels before and at 1, 3, 6, and 12 months after HT to determine their prognostic value on all-cause death or graft failure. Their study provides valuable insight into the potential importance of Gal-3 as an HT biomarker. Suárez-Fuentetaja et al. describe an overall gradual decline in Gal-3 levels 1 year post-HT. However a significant reduction in the biomarker level was demonstrated only after 6 months, while levels measured 1 and 3 months post-HT remained unchanged compared with pretransplant levels.7 These findings support data from previous studies showing persistent elevation of Gal-3 in the short-term following HT.8 The reduction in Gal-3 over a 1-year period may suggest a gradual but progressive reversal with HT of the systemic pathophysiologic, renal and hemodynamic changes of the advanced HF state preceding HT.
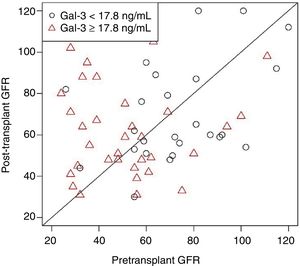
Similarly, other potential biomarkers, such as circulating B-type natriuretic peptide and ST2, have been shown to decline after HT compared with pre-HT levels.6 However, it is important to note that this finding contrasted with that of a prior study by Grupper et al. that demonstrated no change in Gal-3 after HT.6 As discussed below, this finding may have been influenced by differences between the 2 studies in the timing of Gal-3 measurement post-HT. In addition, it has been shown that post-HT Gal-3 levels are higher among patients with elevated pre-HT Gal-3 than among those with lower pre-HT levels (Figure 1). The proportion of elevated pre-HT Gal-3 patients was far greater in the study by Grupper et al. (44%) than in that by Suárez-Fuentetaja et al. (34%), which may account for the overall lack of a significant decrease in Gal-3 levels after HT in the study by Grupper et al.
Galectin-3 levels measured in patients after heart transplant are higher in those patients with elevated Gal-3 levels before heart transplant. The P value was nonsignificant after adjustment for age, body mass index, and pretransplant glomerular filtration rate (GFR). Reproduced with permission from Grupper A et al.6.
An inverse relationship between serum Gal-3 and renal function has been observed in patients with HF9 and HT,6 implying that increased Gal-3 levels in HF might be due to renal dysfunction and that the ability of Gal-3 to predict outcomes in HF might reflect, at least in part, the consequences of renal impairment. The association between elevated Gal-3 levels and renal dysfunction has been demonstrated among HT recipients and is prevalent, since renal dysfunction may persist after HT, especially with the use of calcineurin inhibitors.6 An observation corroborating the close link between renal function and Gal-3 levels was that there was a greater reduction in Gal-3 levels after combined heart and kidney transplant compared with HT alone or combined heart and liver transplant.6 The findings by Suárez-Fuentetaja et al. validate the association with renal function as both univariable and multivariable linear regression analyses identified a significant association between estimated glomerular filtration rate and Gal-3 levels at 1-year post-HT.7 The authors in that study attempted to address the unresolved question regarding the clinical validity of serum Gal-3 as a prognostic biomarker in HT in the context of its relative lack of specificity when adjusted for other clinical parameters, especially renal function. The authors demonstrate that Gal-3 levels at 1-year post-HT are significantly associated with adverse outcomes post-HT, despite adjustment for renal function as measured by estimated glomerular filtration rate as a continuous variable. It is unclear whether this relationship would hold true if the presence of renal dysfunction as a dichotomous variable was included in the multivariable model. This issue is especially relevant, as renal dysfunction after HT is a major independent determinant of morbidity and mortality in this population.10
Another major cause of morbidity and mortality after HT, cardiac allograft vasculopathy (CAV), results from an initial injury to the allograft endothelium causing a chronic inflammatory state and luminal narrowing due to vascular smooth muscle cell proliferation and a fibrotic process that limits beneficial vascular remodeling, with high expression of transforming growth factor (TGF)-β in the intima.11 T cells and macrophages have been shown to modulate the pathogenesis of CAV with a specific role for macrophages regulating the fibrotic response.12 CAV develops in as many as 50% of HT recipients within 5 years of transplant, and vascular fibrosis after HT has been associated with more severe CAV and poor long-term survival.12,13 A previous study by Coromilas et al. showed a significant difference in Gal-3 levels between HT recipients with and without CAV, and higher levels in patients with more severe CAV, suggesting an association between Gal-3 and coronary fibrosis.8 In contrast, the findings by Suárez-Fuentetaja et al. support previous findings from the study by Grupper et al. that posttransplant Gal-3 levels were not significantly associated with the presence of CAV or cellular rejection. Notably, the diagnosis of CAV was based on intravascular ultrasound in the study by Grupper et al., which is a more sensitive modality than coronary angiography, which was used to diagnose CAV in other studies.
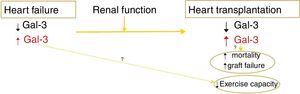
The major finding of the study by Suárez-Fuentetaja et al. is that 1-year posttransplant serum Gal-3 levels were significantly associated with a higher risk of the major composite outcome comprising all-cause death or graft failure over a median follow-up of 6.7 years (interquartile range, 4.3-9.4).7 The association between Gal-3 levels at 1 year post-HT and the study composite outcome remained statistically significant after multivariable adjustment using Gal-3 levels either as a continuous or as a dichotomous variable, less so with the former. However, there was a statistically significant difference in the history of primary graft dysfunction between the high and lower Gal-3 groups, with a higher prevalence not only of a history of primary graft dysfunction but also of elevated right atrial pressures at 1 year in the high Gal-3 HT recipients compared with the lower Gal-3 HT group (29% vs 11%; P=.02 and 11 vs 9mmHg; P=.01, respectively). Whether the independent association of Gal-3 levels with the composite endpoint of all-cause death or graft failure would remain valid after adjustment for a history of primary graft failure is unclear as this variable was not included in the multivariable prognostic model. In addition, the authors do not describe whether there was a difference in the presence of donor DSA pre-HT or de novo DSA developing post-HT. The presence of DSA is an independent and major predictor of long-term major adverse cardiovascular outcomes in HT recipients1,13,14 and should be adjusted for in HT outcome studies. The only other study that evaluated the association between serum Gal-3 levels and long-term morbidity and mortality was the study by Grupper et al., which included a cohort of 62 HT recipients and showed no association between Gal-3 levels and graft function or long-term mortality post-HT during a similar mean follow-up of 6.7 years. The study did show that there was a significant difference in multiple parameters of exercise capacity between the high and low Gal-3 level groups but this difference did not persist when adjusted for age, body mass index, and pretransplant renal function. There was no significant difference between the 2 studies in the event rate as it pertains to mortality. The major difference between the 2 studies, which may explain these conflicting results, is that assessment of outcomes was based on pretransplant Gal-3 levels in the study by Grupper et al. as opposed to 1-year post-HT Gal-3 levels in the study by Suárez-Fuentetaja et al. This occurred in part because of the variability in the timing of acquiring serum samples for Gal-3 measurements post-HT (at 365 days7 vs 365 days [interquartile range, 54-767 days] post HT6). The findings of these 2 studies as it pertains to Gal-3 levels and clinical outcomes are summarized in Figure 2.
The development of more effective immunosuppression therapy has improved post-HT survival and allograft function, but the field remains plagued by a failure to balance immunosuppression to minimize infection and malignancy while preventing acute and chronic rejection. To individualize immunosuppression for each patient, there is a need for more accurate assessment and prediction of individual patient risk for long-term complications post-HT. The data presented by Suárez-Fuentetaja et al.7 represent an attempt to better understand the role of biomarkers to predict outcomes post-HT and highlight the importance of incorporating such biomarkers to fulfill the promise of precision medicine. The results presented in this study will likely need prospective validation in a multicenter setting after adjustment for variables such as DSA, but it is an important first step in an attempt to identify a post-HT biomarker that could be incorporated in clinical practice. Currently, a single biomarker is unlikely to be of clinical usefulness in HT as evidenced by the relatively low sensitivity (57%) and specificity (75%) of Gal-3 for death or graft failure. A multivariable model would have to be developed incorporating clinical, imaging, and biochemical risk factors to improve the predictive value of such testing.
FUNDINGN.L. Pereira is funded in part by the National Institute on Aging grant R21AG53512.
CONFLICTS OF INTERESTNone declared.