Keywords
INTRODUCTION
Acute aortic syndrome is an acute lesion of the aortic wall accompanied by weakening of the media which increases the risk of aortic rupture and other complications, and has high morbidity and mortality. It has three components: aortic dissection, intramural hematoma, and penetrating ulcer. It has an incidence of 30 cases per million per year, of which 80% are aortic dissections, 15% intramural hematomas, and 5% penetrating ulcers.
Advances in imaging techniques have both significantly improved the diagnosis of acute aortic syndrome and yielded fundamental information regarding the evolution of this disease. On the other hand, the development of new surgical techniques and the advent of endovascular treatment have modified the therapeutic strategy and prognosis.
AORTIC DISSECTION
Aortic dissection is the most frequent and serious form of acute aortic syndrome, with more than 60% mortality in the first week of evolution if suitable treatment is not rapidly begun. In an exhaustive review of the literature, Hirst et al1 reported that in a total of 505 cases mortality in the first 24 h was 21%, at 48 h 38%, at 7 days 62%, and at 14 days 74%. In order to improve prognosis in these patients, high clinical suspicion is required in the presence of clinical symptoms or signs, especially in hypertensive patients, those with Marfan syndrome, or with atherosclerotic aneurysms. In these cases, imaging should be done as soon as possible to either confirm or reject the diagnosis and begin immediate treatment. The ascending aorta is affected in 62% of cases (type A) and unaffected in 38% (type B).
Type A Aortic Dissection: Evolution and Treatment in the Acute Phase
The most characteristic clinical symptoms of type A aortic dissection are anterior thoracic pain (85%) and/or back pain (46%), abdominal pain (22%), syncope (13%), and stroke (6%).2 Numerous studies have confirmed the high early mortality of type A aortic dissection compared to type B.1-4 Despite diagnostic and therapeutic progress, mortality during hospitalization for type A aortic dissection was 33% in the International Registry of Acute Aortic Dissection (IRAD) series.5 The results obtained from this series make it possible to obtain a model which predicts mortality during hospitalization. By applying this model, renal failure has a score of 1.6, hypotension/shock/tamponade 1.1, sudden initial pain 1, and pulse deficits 0.6. A global score of 1 is associated with 10% mortality, 2.5 with 35% mortality, and 4 with 65% mortality.
Type A aortic dissection requires urgent surgical treatment, particularly when the patient presents within the first 48 h, unless there are formal contraindications, and especially if there are serious irreversible cerebral or visceral injuries. The current trend is to prevent such complications, especially with intravascular techniques via balloon fenestration and stent implant, which potentially strongly improves the prognosis of the patient. Early restoration of reperfusion to the vital organs makes it possible to stabilize the patient, such that surgical repair can be done with increased chances of success.6 In teams with wide experience, perioperative mortality was only 15%.7 Nevertheless, in studies which included reference centers with teams with intermediate experience, surgical mortality was 26%.2 Mortality was 58% in the group medically treated for type A aortic dissection, due to comorbidity or refusing surgery.
The main limitation to the urgent surgical treatment of type A aortic dissection can be the surgeon's experience in the treatment of aortic disease. Clearly, this involves complex surgery requiring a high level of surgical expertise which cannot always be offered by the team on duty. In this sense, a possible solution would be to have expert surgeons on call or to select reference centers for aortic disease in each geographical area.
During surgical treatment, establishing extracorporeal circulation with anterograde arterial perfusion is recommended, either via the axillary or the right subclavian route, or even via the ascending aorta itself, confirming by transesophageal echocardiography that the true lumen has been perfused. Retrograde femoral perfusion is not free from complications, which may include embolization of atheromatous material, extension of the dissection or even hypoperfusion of the organs. Surgery done with total circulatory stoppage involves the use of meticulous protection methods, with deep hypothermia and selective cerebral perfusion. Circulatory stoppage for more than 30-40 min gives rise to cerebral, respiratory and coagulation complications. Thus, the combination of cerebral perfusion techniques with anterograde hypothermia is currently becoming stronger.7 In a recent study published by Ehrlich et al,8 out of a total of 167 patients who underwent surgery between 1987 and 2001, 31% died or presented permanent neurological deficit, which was only related to hemodynamic instability (odds ratio [OR]=6.0). On the other hand, 16% of patients had transitory neurological injuries that were related to being more than 60 years old and having associated coronary disease.
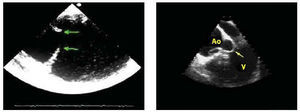
Surgical treatment basically consists in closing the dissection entry port and replacing the ascending aorta and aortic arch if needed, having repaired and reconstructed the distal aorta beforehand. Repair of the aortic root and preservation of the aortic valve should always be attempted using David's9 or Yacoub's resuspension technique.10 However, if anatomical injury to the aortic root is very serious, to avoid reinterventions, it is better to carry out complete replacement with a valved conduit with coronary artery reimplantation. This is especially true in cases of ectasia of the aortic root or in Marfan syndrome.11 Anastomosis of the most distal part of the prosthetic tube with the ascending aorta wall is suitable whenever there is no entry port in the aortic arch. The entry port is located in the arch in approximately 30% of patients and in these cases the arch should be replaced beyond the entry port.12 If there is an aneurysm in the proximal descending aorta, dissected or not, extending an elephant trunk from the arch is a good option.13 In a later procedure a prosthetic tube placed in the descending aorta can be connected distally. Some surgical teams proceed, during the same intervention, with implantation of a stent in the distal aorta with an open technique, to prevent potential complications in this location. The role of transesophageal echocardiography (TOE) should be emphasized regarding optimizing the results of the various surgical strategies.14 The technique provides precise information on the location of the entry port, etiopathogenesis of aortic incompetence, and disorders of the distal arch and descending thoracic aorta15 (Figure 1).
Fig. 1. Transesophageal echocardiography. In the left-hand image a large entry port distal to the aortic arch can be seen. In the right-hand image the prolapsed intimal flap can be seen within the left ventricle (arrow) through the aortic valve, demonstrating the etiology of severe aortic insufficiency. Ao indicates aorta; V, left ventricle.
Twenty-five per cent of type A aortic dissections are retrogressive, that is, with an entry port in the arch or descending aorta. In a recent study, Kaji et al16 showed that retrograde thrombosed type A aortic dissection has a better prognosis than anterograde, both in the acute phase, with a mortality of 15% versus 38%, as well as at 5 years, with mortality of 15% versus 43%. These authors recommend medically treating retrograde type A dissections with total thrombosis of the false lumen and surgical intervention in retrograde dissections with flow. We believe that further studies should confirm these results, although it would be useful to have such information for patients older than 75 years or with comorbidity.
Evolution and Long-Term Treatment
Even though surgery completely changes the natural history of type A aortic dissection, those who survive surgical treatment can develop complications. Basically, there are two categories: failure of the proximal repair or failure of the distal aorta. Failure of proximal repair tends to be the result of a poor surgical decision or is due to the technique used for reconstructing the aortic root and the valve. Progress in surgical techniques has strongly reduced complications arising from proximal failure. However, complications due to failure of the distal aorta and the persistence of false lumen flow have not changed significantly. Several studies have related the persistence of false lumen flow to the evolution of an aneurysmatic dilatation of the distal aorta, late reintervention and death due to aortic rupture.17-21 Persistence of false lumen flow ranges between 79% and 90%.1821 Although persistence of false lumen flow of the descending aorta has been considered unavoidable and cannot be considered a complication, morbidity and long-term mortality relating to this situation are high.21 The physiopathological mechanism is due to flow because of a persistent entry port in the arch or proximal descending aorta, or because of incorrect suture of the prosthetic tube to the aorta wall. Thus, the elimination of the proximal entry port should be a high priority in surgical intervention. It is important to resect all the communication ports proximal to the left subclavian artery; in these circumstances, replacing the arch can diminish the persistence of false lumen patency from 60% to 25%. Stent-graft placement diminishes the incidence of distal false lumen patency more than suturing the segments of the terminal aorta.
Medical treatment after surgery is fundamental. Correct control of blood pressure is related to 96% survival free from rupture at 5 years, compared to 61% in the poorly controlled group. Systolic blood pressure should not exceed 100-110 mm Hg, without exception. Follow-up with imaging techniques is important to screen for the appearance of a new dissection or the formation of aneurysms in other sites of the aorta. The incidence of such recurrence is approximately 25%, frequently evolving with complications such as rupture and death by bleeding.
A TOE should be done after surgery at follow-up, and computerized tomography (CT) or magnetic resonance (MRI) at 6 months and 12 months. This would subsequently be done annually providing the patient remains stable and asymptomatic.
Survival free from complications in type A dissections treated surgically is 84% at 5 years when the false lumen is totally thrombosed and 63% when flow is observed in the false lumen.21 Other extensive series demonstrate that the main markers of late mortality are being more than 70 years old and the presence of false lumen patency after surgery.17
Type B Dissection: Evolution and Treatment in the Acute Phase
Dissection that affects the descending aorta has a similar clinical presentation to type A dissection, although the sudden initial pain is located in the back and abdomen in 64% and 43% of patients, respectively. Cerebrovascular accidents and syncope are less frequent.22
Patients with uncomplicated type B dissection have a 30-day mortality of 10%.2 In contrast, mortality is 30% in those who develop ischemia in the legs, renal failure, visceral ischemia, or contained rupture frequently requiring urgent aortic repair.
Data from the International Registry of Aortic Dissection (IRAD)22 showed that 12% of type B dissections presented hypotension or shock, aortic diameter was greater than 60 mm in 16% of the cases, there was periaortic hematoma in 19% of cases and arterial vessel disease with bad perfusion in 22% of cases, mainly in the ileac, mesenteric or renal arteries. Hospital mortality was 13% and the majority of deaths occurred in the first week. The variables associated with the increase in mortality were complications in the acute phase: coma/altered consciousness (58%), hypotension/shock/tamponade (47%), need for surgical treatment (32%), mesenteric or limb ischemia (28%), aorta diameter >60 mm (27%), periaortic hematoma (26%), acute renal failure (22%), and mediastinal enlargement (16%). A mortality risk model demonstrated hypotension/shock, the absence of thoracic pain at clinical presentation and branch vessel involvement to be a high-risk triad. This model, together with age greater than 70 years and male sex, reveals three groups with marked differences in mortality, 3%, 36%, and 71%, respectively. The lack of thoracic pain (14%), almost always due to presenting shock or syncope, constitutes a risk factor due to the difficulty involved in making a diagnosis, since 50% are diagnosed 6 h after the acute event and 30% 2 days after presentation. Other studies have shown that age, hemodynamic compromise, and peripheral ischemia are the variables with worse prognostic value in type B aortic dissection.4,23 The prognostic value of total thrombosis of the false lumen continues to cause controversy.24
Medical Treatment
Patients with type B dissection should receive immediate treatment to control the pain and lower systolic blood pressure to 110 mm Hg. With this aim, morphine sulphate and intravenous beta-blockers (metoprolol, propranolol, or labetalol) can be used, or vasodilator drugs such as nitroprusside or ACE inhibitors. If beta-blockers are contraindicated, calcium antagonists such as verapamil or intravenous diltiazem can be used. In normotensive or hypotensive patients it is important to check for blood loss, pericardial effusion or heart failure. In patients with severe hemodynamic instability, intubation, mechanical ventilation, and a TOE or a CT should be done.
Surgical Treatment
Surgery for type B dissection is indicated when the patient's life is endangered by some of the aforementioned complications: hemodynamic instability, intractable pain, rapid expansion of aortic diameter, and mediastinal or periaortic hematoma as signs of imminent rupture of the aorta. Dissection of an pre-existing aortic aneurysm can also be considered a surgical emergency, as can the appearance of signs of bad perfusion of the key arterial branches. Surgical repair of the dissected thoracic aorta can reverse 90% of pulse deficits. However, patients with mesenteric or renal ischemia do not present the same reversibility. Patients with renal ischemia have a mortality of 50%-70% and those with mesenteric ischemia, 80%-90%.25,26 A review of the literature shows that surgical mortality in type B dissection is very high (28%-65%).23,27 In addition, around a third of patients (30%-35%) have a risk of paraplegia. Mortality due to surgical fenestration is high (20%-60%), which is why percutaneous treatment for hypoperfusion has been suggested as a good alternative before surgery.
Percutaneous Fenestration
The indications for percutaneous fenestration are not well-established and this procedure has become even less common since endovascular treatment has become available. In patients with arterial ischemia due to the false lumen compressing the arterial ostia, it is essential to decompress the false lumen by closing the entry port by stenting or, if this is not possible or effective, carrying out fenestration of the infrarenal aorta thereby creating an exit port in the false lumen. Intravascular balloon fenestration is especially indicated for treating mesenteric ischemia.28,29 Intravascular ultrasonography can be of great help in obtaining optimal results with this technique.
Endovascular Treatment
Endovascular treatment has recently been introduced for treating aneurysms of the descending thoracic aorta and type B aortic dissection. In 1999, Dake et al30 and Nienaber et al31 simultaneously published their initial experiences with endovascular treatment in 19 and 12 type B dissections, respectively. The false lumen is excluded from the circulation by closure of the entry port and development of aneurysmatic dilatation and later rupture of the aorta is thus avoided.32 Endovascular treatment not only helps to dramatically reduce mortality due to type B dissection, but it can probably change the natural history of this disease. Considering the excessive surgical mortality in patients with bad perfusion of the arterial branches, percutaneous treatment is suggested to improve perfusion followed by surgical treatment, if necessary. On the other hand, most postimplant complications arising from the intravascular prosthesis are related to the severity and duration of the ischemia before the procedure.33 Currently, it could be accepted that endovascular treatment is indicated when there is a static or dynamic obstruction of one of the arterial trunks. Static obstruction might be solved by placing a stent at the origin of the vessel, and dynamic obstruction by decompressing the false lumen by closing the entry port or by fenestration. Other indications are rapid dilatation of the aorta and indirect signs of aortic rupture, although no series validates this treatment with regard to surgery in the acute phase.
Evolution and Long-Term Treatment
Long-term survival regarding ty pe B dissection has been considered similar to that of surgically treated type A dissection, i.e. 75% at 5 years.4,34 Adequate medical treatment with beta blockers is fundamental to prevent complications. With the reduction of blood pressure and dP/dt, beta-blockers delay the expansion of the aorta. Doses should be increased progressively until pressures lower than 130/80 mm Hg have been attained. Some follow-up studies of type B dissection report an 18-30% risk of aortic rupture at 3-5 years, and a 20%-30% need for surgical treatment.23,35 Predictive factors are age, chronic obstructive pulmonary disease, hypertension and the basal maximum diameter of the aorta.23 Griepp et al35 did not find a relationship between aortic diameter and rupture in a series of 50 patients with type B dissection, at an average follow-up of 40 months. The explanation could be that patients with greater aortic diameter had undergone surgery. Nevertheless, it is important to point out that the average final value of aortic diameter in patients who died of rupture was 54 mm.
Follow-up of type B dissection with imaging techniques has made it possible to know how aortic diameters evolve as well as various epiphenomena, such as thrombosis of the false lumen, compression of the true lumen and arterial trunks or reextension of the dissection (Figure 2). Imaging is advisable at 3 and 6 months following the acute episode, and annually thereafter. In an interesting study, Kato et al36 demonstrated that an aortic diameter greater than 40 mm in the acute phase and the presence of a patent entry port in the thoracic aorta are markers of the development of an aneurysm in the false lumen (>60 mm). In this group of patients, 30% had an aneurysm of the aorta at 3-year follow-up. In patients with an aortic diameter less than 40 mm or greater than 40 mm, the diameter increased by 0.2 mm per year and 0.8 mm per year, respectively. Other studies agree that maximum aortic diameter is the variable with the greatest prognostic value regarding complications during follow-up.4 Marui et al37 found that in 67% of the patients who had an aortic diameter >40 mm and a patent false lumen, the disease evolved into severe dilatation or aortic rupture at 5 year follow-up, whereas this only occurred in 6% of patients who did not present these 2 variables during the acute phase. These data suggest that the subgroup of patients in whom closure of the entry port is more beneficial should be identified to carry out surgical treatment before hospital discharge. In the remaining patients blood pressure should be strictly controlled with beta blockers and if serious dilatation of the aorta >3 mm/year is detected, surgical or intravascular treatment is advisable which, in any case, is indicated in aortas with a diameter greater than 60 mm. Considering that surgical mortality in type B dissection in the subacute or chronic phase can be 10% and the risk of paraplegia 7%,35 endovascular treatment is an attractive alternative (Figure 3).
Fig. 2. Type B aortic dissection followed up via computerized tomography. At 2-year follow-up, a 16-mm increase in the maximum diameter of the aorta can be seen.
Fig. 3. Left-hand image: magnetic resonance reveals a type B aortic dissection with serious dilatation of false lumen in the proximal part of the descending aorta. Right-hand image: the false lumen has been correctly excluded after implantation of a stent in the proximal part of the descending aorta. The false lumen has remained completely thrombosed up to the height of the celiac trunk which is retrogressively filled from the false lumen.
Recent studies demonstrate that endovascular treatment for dissection of the descending aorta is safe and yields better results than surgery.30,31 The incidence of complications is clearly lower, being 4%-7% for vascular accidents and 2%-3% for paraplegia. Stroke seems to be related to the manipulation of catheters in the aortic arch, and paraplegia to stent length (>16 cm), previous or simultaneous surgery of the aorta and perioperative hypotension. The yearly results are excellent, with a survival higher than 90%, thrombosed false lumen and no increase in the size of the aorta. It remains to be determined whether implantation should be done in all dissections or just in the subgroup which presents poor clinical evolution or development of aortic aneurysm in the subacute or chronic phase. Taking into account that 15%-30% of type B dissections require surgical treatment during evolution, an attractive alternative would be to carry out endovascular treatment in the acute phase in all patients with patent false lumen and an aortic diameter greater than 40 mm. Nevertheless, we should note that in 20%-30% of patients treated in this way, flow in the false lumen persists30,33 and the long-term evolution remains unknown.
INTRAMURAL HEMATOMA
Aortic intramural hematoma has been regarded as a precursor of aortic dissection; however, the physiopathological mechanism, evolution and prognosis are quite different. Intramural hematomas originate from the spontaneous rupture of the vasa vasorum or a penetrating atherosclerotic ulcer. Although the clinical presentation of aortic dissection and intramural hematoma are quite similar, recent progress in imaging techniques has helped in the diagnosis of the latter, which forms between 13% and 30% of the acute aortic syndrome.38-41 Intramural hematoma affects patients with more atherosclerotic risk factors and older age than aortic dissection, occurring in 70% of cases in the descending aorta. Intramural hematoma is diagnosed with similar accuracy with TOE, CT, or MRI.42 Selection of the imaging technique depends on the availability of each center and staff expertise. MRI has an advantage over other imaging techniques because it can detect acute or chronic bleeding.
Evolution and Short-Term Treatment
Intramural hematomas have a very dynamic evolution and can be reabsorbed, progress to a classical or localized dissection, or present a contained rupture during the first days of evolution.40,42
Early mortality basically depends on location and is higher when it affects the ascending aorta.40,43 However, there are large differences in the published results, with 36% mortality for medically treated type A hematomas in a metaanalysis of European and American publications,41 and less than 10% in the majority of Asian groups.44,45
Although the therapeutic strategy for intramural hematomas should be the same as for aortic dissection,46 some type A subgroups--particularly in the absence of dilatation of the ascending aorta and with a hematoma thickness less than 11 mm--can be reabsorbed with medical treatment. Some studies have shown that intramural hematomas of the ascending aorta, when the size of the aorta is less than 50 mm, tend to be reabsorbed, whereas, if the diameter is greater than 50 mm, they tend to progress and evolve into dissection or aortic rupture.41,43-45 The most effective therapeutic strategy for intramural hematoma of the ascending aorta has still to be definitively established. Nevertheless, in view of the fact that the risk of mortality seems lower than that of type A aortic dissection, treatment with beta-blockers should be initiated and nonurgent surgical intervention be performed, except in situations of hemodynamic instability or signs of imminent rupture (Figure 4). The subgroup of patients with intramural hematomas with a thickness less than 11 mm in the ascending aorta, a maximum aortic diameter less than 50 mm, and an absence of associated complications, can benefit by careful follow-up with imaging every 2-3 days44 (Figure 5). Only cases clearly evolving toward reabsorption that do not present any complications can be managed with medical treatment.47
Fig. 4. Intramural hematoma of ascending aorta that progressed with dissection of the descending aorta. Upper images: magnetic resonance carried out in the acute phase revealed a large intramural hematoma in the ascending aorta (left) and a dissection of the descending aorta (right). Lower images: magnetic resonance at 6-year follow-up shows (left) a successful surgical outcome in the ascending aorta with persistent dissection of the descending aorta. The right lower image highlights an aneurysmatic dilatation with partial thrombosis of the right ileac artery.
Fig. 5. Computerized tomography shows an intramural hematoma affecting the ascending and descending aorta (left). At 3-month follow-up (right), the hematoma of the ascending aorta has been reabsorbed totally without aortic dilatation and has evolved into a classical dissectio by the Stanford group48 it was found that hematomas associated with images of aortic ulcers progressed or presented complications, especially when they were located in the ascending aorta or arch, and when the ulcer had a diameter greater than 20 mm or was deeper than 10 mm. The main limitations of this study are that it was retrospective, did not include a follow-up protocol and the frequency of penetrating atherosclerotic ulcer was strikingly high (52%). In our study, by using TOE and MRI, we found that many of the images that resemble an ulcer are localized dissections with a well-defined intimal flap and a main entry port.
It is well known that the incidence of periaortic hematoma and pleural effusion is higher in intramural hematoma than in dissecting hematoma.43,44 These complications would not indicate surgical treatment by themselves if progression was absent or if they were not accompanied by other poor prognosis factors.
Type B intramural aortic hematoma should be treated medically like type B dissection, except in cases where there is severe dilatation of the aorta (>60 mm), signs of imminent aortic rupture or bad clinical-hemodynamic evolution.
Unlike aortic dissection, where most complications occur during the acute phase of the event, intramural hematoma can evolve in different ways and present possible complications during the subacute phase and the first 6 months. For this reason, it is essential to use at least one or two imaging techniques during the subacute phase and before hospital discharge.
Evolution and Long-Term Treatment
Mortality and Late Progression
The long-term prognosis of type A hematoma is clearly better than that of aortic dissection. In the series of Kaji et al,49 17 (57%) patients out of 30 with hematomas of the ascending aorta were discharged without surgical treatment. The thickness of the hematoma decreased from 9±3 mm to 1±3 mm and the maximum diameter of the ascending aorta from 48±5 to 45±6 mm. Total resolution of the hematoma was observed in 40% of the patients and there was no mortality at 56±37 months follow-up. In our experience, 2 out of 8 medically treated patients evolved into a dissection free of complications after 3-month follow-up. Thus, follow-up must be done every 2 or 3 months with imaging techniques during the first year.
Hematoma of the descending aorta also has a better prognosis than dissecting hematoma. Asian research groups have reported 5-year survival in 97% of cases of intramural hematoma versus 79% for dissection of the descending aorta.50 In our series, mortality in patients with aortic hematoma was 19% at 3 months and 34% at 5 years, which is very similar to that of other non-Asian groups.43 In a recent publication, von Kodolitsch et al46 reported that late progression of intramural hematoma was associated with younger age (49±17 years vs 64±11 years in intramural hematoma without progression; P<.003) and the absence of beta-blocker treatment; only 7% of intramural hematomas with late progression were treated with beta-blockers compared to 49% of hematomas without late progression (P=.009).
Long-Term Morphological Changes of Intramural Hematoma
There are few studies on the morphological evolution of the aorta wall after intramural hematoma. In our series51 of 50 intramural hematomas followed up with imaging techniques over 45±31 months, we demonstrated that evolution is very dynamic, mainly in the first 6 months. Regarding long-term evolution, we showed that intramural hematomas regress without complications in 34% of cases, in 36% they progress to dissection, in 12% to classical dissection, in 24% to localized dissection, and in 30% they evolve into fusiform or saccular aneurysm. One of the most striking aspects of the evolution of intramural hematoma is its evolution into a localized dissection (Figure 6). After some months, the small intimal flap decreases in size or disappears and the lesion, depending on size, looks like an aortic ulcer or a pseudoaneurysm. Many authors consider such images to be serious complications of intramural hematoma,48,50 but in fact some disappear without complications50,51 whereas others tend to dilate.51 The evolution of this long-term complication should be assessed regarding possible endovascular treatment, although, in any case, poor prognosis has not been demonstrated in the short to medium term. Another noteworthy aspect of our study was the progression to saccular aneurysm from a small atherosclerotic aortic ulcer that evolves with asymptomatic rebleeding of the wall, which was only detected with MRI. In one of these cases, the aorta expanded from 40 mm to 96 mm over 7 years without the patient presenting any symptom.
Fig. 6. Computerized tomography (CT) shows an intramural hematoma of the descending aorta (upper left). At 6 months, the hematoma evolves with a localized dissection (upper right). Spiral CT (lower left) shows an image of ulcerlike lesion secondary to the hematoma communicating with the aortic lumen due to a localized dissection (lower right).
In our experience,51 the maximum aortic diameter in the acute phase was the variable with greatest prognostic value regarding reabsorption without dilatation and other complications. The group that showed such good evolution had a maximum aortic diameter in the area of the hematoma clearly smaller than the group that developed complications (39±4 vs 47±7 mm) and thinner intramural hematoma (12±4 vs 14±4 mm; P<.05). The variables relating to evolution into aortic dissection (classical or localized) were echolucency (78% vs 34%; P<.02) and hematoma length (94% vs 63%; P<.01). Patients who developed fusiform or saccular aneurysm (Figure 7) more frequently had an atherosclerotic disease in other cardiovascular sites (67% vs 23%; P<.05) and a higher prevalence of ulcerated atherosclerotic plaque (47% vs 9%; P<.005).
Fig. 7. Intramural hematoma of the descending aorta diagnosed by magnetic resonance (upper images) that evolves into fusiform aneurysm (lower images).
Given the dynamic evolution of the intramural hematoma and that 15%-20% of the patients die of aorti c rupture in the first 5 years, a very careful follow-up at 3, 6, and 12 months is recommended, and subsequently every year until the intramural hematoma has been totally reabsorbed. Patients with dilatation of the aorta or images of an ulcer on the aorta wall should be followed up closely a nd treated more aggressively through surgery or endovascular treatment. If the evolution shows a progressive increase in aortic diameter, thickness of the hematoma or signs of rebleeding in the aorta wall, surgical or endovascular treatment is indicated. The usefulness of endovascular treatment in the acute phase has not been validated and can give rise to complications due to rupture of the intima at the extremes of the stent. Unless the hematoma is very localized, it is advisable to wait at least 3-6 months so that the bleeding in the wall evolves into fibrosis and the wall is less friable than in the acute phase.
AORTIC ULCERS
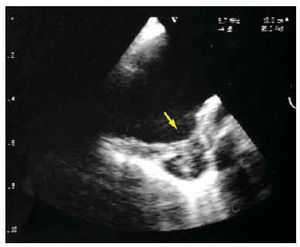
Less information is available on the acute aortic syndrome. The diagnosis of an aortic ulcer obtained via imaging techniques can correspond to various processes, with different pathogenesis and prognosis. Basically, aortic ulcers can correspond to ulcerated atherosclerotic plaque, penetrating atherosclerotic ulcer or to images of ulcer in the aorta wall secondary to the evolution of other aortic diseases (similar to ulcers). Contrast imaging techniques, such as angiography and CT, which make it possible to visualize the protrusion of an ulcer from the aortic lumen, have greater sensitivity regarding the diagnosis of aortic ulcers. Nevertheless, it is frequently impossible to differentiate the pathogenesis of different types of ulcer with these techniques. In our experience, TOE has low sensitivity for the diagnosis of aortic ulcers, but is the technique of choice to classify the different types of ulcers with regard to their pathogenesis. Visualization of the aortic lumen and wall is excellent via this technique and makes it possible to obtain a differential diagnosis between the ulcerated plaques observed above the intima and the atherosclerotic ulcers that penetrate the intima in the media and frequently deform the adventitia (Figure 8). Finally, TOE is very useful for diagnosing ulcerlike lesions that are observed in the evolution of aortic intramural hematoma as a consequence of localized dissection or pseudoaneurysm, or for ulcer secondary to a crater in the surface of a parietal thrombus.52
Fig. 8. Penetrating atherosclerotic ulcer (arrow) in the descending aorta diagnosed through transesophageal echocardiography.
Differentiation of the various types of aortic ulcer is important, since their evolution and prognosis are different. Ulcerated atherosclerotic plaque is not accompanied by symptoms and is usually an accidental finding during TOE; its evolution is not well understood, although some can evolve into a penetrating ulcer. Acute aortic symptomatic penetrating atherosclerotic ulcer has a risk equal to or higher than that of acute aortic dissection or intramural hematoma. Penetrating ulcer is normally diagnosed in patients over 60 years old with atherosclerosis in other areas and related cardiovascular risk factors. Like intramural hematoma, it is located much more frequently in the descending aorta.53 The most extensive series published report an incidence of aortic rupture of 10-40%.54,55 In some cases this evolves into saccular or fusiform aneurysm.51 On the other hand, it is well known that many penetrating ulcers are accompanied by intramural bleeding and are surrounded by intramural hematoma. In our experience, the majority of penetrating atherosclerotic ulcers in the acute phase are diagnosed in the context of an intramural hematoma. However, its diagnosis is not exceptional in asymptomatic patients. After the acute phase, penetrating ulcers can remain completely stable or progress to dilatation, often with asymptomatic rebleeding of the wall. MRI makes it possible to assess the presence of rebleeding in the aorta wall that would call for a more aggressive treatment.
Treatment
The treatment of symptomatic penetrating atherosclerotic ulcer in the acute phase should be similar to other acute aortic syndromes, given the risk of aortic rupture.53 After the acute phase, treatment depends on the pattern of evolution, and its effect on symptoms, progressive dilatation, or rebleeding of the aortic wall. When endovascular treatment was not available, surgery involved a significant risk in senile patients and those with atherosclerosis, and it was often found that many aortic ulcers remained totally stable in the short-medium term. There is no information in the literature on the evolution and prognosis of such aortic ulcers in the long-term. Nevertheless, we believe that in larger ulcers, especially if they are dilating or causing rebleeding in the aorta wall, endovascular treatment may be indicated.
CONCLUSIONS
Acute aortic syndrome continues to yield high mortality despite diagnostic and therapeutic progress during the last decade. Better understanding of the natural history and prognostic variables of this disease can be of great assistance regarding the most suitable therapeutic strategy for its treatment. However, clinical suspicion and the surgeon's expertise continue to be the basic key factors affecting early mortality. The advent of endovascular treatment has opened new perspectives in the management of acute aortic syndrome affecting the descending aorta, since this can modify its natural history and improve prognosis.
ACKNOWLEDGMENT
My thanks to Herminio García del Castillo for his suggestions in drafting the text.
Correspondence: Dr. A. Evangelista Masip.
Servei de Cardiologia. Hospital Universitari Vall d'Hebron.
P.o Vall d'Hebron, 119-129. 08035 Barcelona. España.
E-mail: evangel@hg.vhebron.es