Keywords
INTRODUCTION
Heart disease is the single most important cause of death. Even though the number of deaths due to ischemic heart disease and cerebrovascular disease has fallen in recent years we have simultaneously witnessed an increase in the prevalence, morbidity and mortality of heart failure.1,2 Heart failure can be the final phase of many illnesses (coronary heart disease, hypertension, valvular heart diseases, cardiomyopathy). Improved treatment and increased survival of patients with these illnesses and the progressive aging of the population have led to the growing prevalence of this enormously debilitating illness.
In the United States, the prevalence of heart failure is estimated at 1% (0.3%-2%)3,4 in the population at large, but increases with age (5%-10% at >75 years). Heart failure is the most frequent cause of hospital admissions in patients aged 65 years, with a mean stay of 7 days, or 13 days if intensive care is required. Readmissions in the first year stand at 13%.5 In Spain, heart failure and ischemic heart disease are the two most frequent causes of admission in cardiology and account for 3.7% of admissions in patients >45 years. Mean hospital stay ranges from 9.5 to 13 days.6
Worldwide, the prognosis for heart failure is poor due to the progress of the illness and the high incidence of sudden death. Four-year mortality is 20%-30% among patients with mild, asymptomatic heart failure and 30%-40% among those with mild-to-moderate heart failure; first year mortality is 50% among patients with severe heart failure.7,8 Despite drug treatment with beta-blockers, angiotensin converting enzyme inhibitors, spironolactone, digoxin, and diuretics, the prognosis for patients with severe heart failure is still rather poor in terms of mortality and the substantial handicaps the illness entails.
CONDUCTION DISTURBANCE IN THE BUNDLE OF HIS-PURKINJE: CLINICAL AND HEMODYNAMIC CONSEQUENCES
Complete left bundle branch block has a range of consequences,9,10 as shown in Figure 1 (Yu et al10 adapted). Firstly, it disturbs left ventricular synchrony, one result of which is decreased cardiac contractility (dP/dT) leading to reduced ejection fraction, a fall in cardiac output, a consequent worsening in hemodynamic condition and, therefore, functional deterioration. This intraventricular asynchrony causes or augments mitral insufficiency, perhaps due to disturbed papillary muscle function, and slows lateral wall contraction. Secondly, atrioventricular asynchrony stems from the disturbed cardiac conduction system: the atrium contributes less to ventricular filling which increases left atrial pressure and left ventricular end-diastolic volume. Prolongation of systole leads to reduced filling time which can cause or worsen diastolic dysfunction. Finally, interventricular asynchrony appears, reducing right ventricular ejection volume and worsening the patient's overall condition.
Fig. 1. Mechanisms of improvement after cardiac resynchronization. LA indicates left atrium; LVEF, left ventricular ejection fraction; CO, cardiac output; MI, mitral insufficiency; LV, left ventricle; RV, right ventricle; LVESV, left ventricular end-systolic volume; LVEDV, left ventricular end-diastolic volume; EV, ejection volume. Adapted from Yu CM et al10.
Electrical disturbances caused by complete left bundle branch block have a series of mechanical and hemodynamic consequences that ultimately produce ventricular remodeling. This makes the heart behave inefficiently and leads to further deterioration in clinical condition. Thus, among patients with the same degree of heart failure those with a greater level of conduction system disturbance present greater mortality11.
Approximately 15% of patients with heart failure present delayed inter- or intraventricular conduction.12,13 This happens in up to one third of patients with moderate-to-severe heart failure.14-16 Patients with heart failure and depressed ejection fraction who also have complete left bundle branch block present an even greater decrease in contractility for various reasons (uncoordinated left ventricular contractility, septal contraction anomalies, disturbed ventricular filling, increased mitral insufficiency) which increase mortality.16
Both left bundle branch block and chronic right ventricular apex pacing can have negative effects on ventricular function. One of the MOST substudies17 shows that ventricular desynchronization caused by chronic right ventricular pacing in patients with diseased sinus node and normal QRS duration increases the risk of hospitalization for heart failure and atrial fibrillation even though atrioventricular synchrony is maintained. Similar data came from a MADIT II sub-study which also confirmed that patients receiving chronic right ventricular pacing were at greater risk of adverse events. Results of the DAVID study18 can be interpreted in a similar fashion showing a worse prognosis in patients with dual-chamber versus single-chamber defibrillators.
THE BEGINNINGS OF STIMULATION TO TREAT HEART FAILURE
In 1983, more than 2 decades ago, de Teresa and Chamorro19 followed by Lorenzo Silva in his doctoral thesis, proved that ejection fraction improves when stimulation originates in the left ventricle and that biventricular stimulation enhances this improvement. In the late eighties, Hochleitner et al20 described how patients improved after the implantation of a dual-chamber pacemaker with slight atrioventricular delay. These studies included few patients and focused on acute phase outcomes. Moreover, results varied greatly. In 1985, Linde et al21 reported on patients with cardiomyopathy and pacemakers that optimized atrioventricular interval. Although patients presented some initial hemodynamic improvement, this had disappeared at 3 months. Gold et al22 found no acute phase improvement in patients implanted with VDD pacemakers programmed with different atrioventricular intervals. The different etiologies of the original cardiomyopathy, different New York Health Association (NYHA) functional classes, left ventricular ejection fraction, PR interval duration, or differences in follow-up may have led to such disparate conclusions.
Currently, the ventricular electrode of dual-chamber pacemakers is sited at the right ventricular apex for greater simplicity. However, this produces a series of alterations, with a change in electric impulse conduction similar to that which occurs in complete left bundle branch lock, which result in reduced cardiac contractility. Some studies have tried to remedy this by placing the electrode in the outflow tract, meaning that the impulse travels downwards and ventricular depolarization is more physiological. Despite this stimulating theory, conclusions prove contradictory. Unfortunately, most studies only analyze acute phase patients, and the only exception lasted just 7 months.23,24
TECHNICAL ASPECTS OF BIVENTRICULAR PACEMAKER IMPLANTATION
In clinical terms, the most important and sometimes most difficult aspect of biventricular pacing is electrode placement in the left ventricle to improve ventricular synchrony (Figure 2).
Fig. 2. Radiography of thorax in posteroanterior (A) and lateral (B) projection, which shows electrode sites in the right atrium, right ventricular apex, and posterolateral vein.
Initially, electrodes to stimulate the left ventricle were implanted by thoracotomy or thoracoscopy to provide stimulation from the epicardium. Intervention time and adverse event rates have been reduced with the development of new electrodes, the use of the coronary sinus to stimulate the left ventricle and the electrophysiologists' learning process. Auricchio et al25 achieved 85%-90% success in coronary sinus cannulation. One problem with this procedure is the anatomic variety among patients and the presence of valves that make access difficult. Physicians require an adequate method of exploration that provides high resolution images and facilitates imaging in oblique positions. Diagnostic and/or steerable electrophysiological catheters are invaluable in coronary sinus catheterization and it may be necessary to use an intracavity electrogram to locate the coronary sinus ostium.
After coronary sinus cannulation has been achieved, the appropriate vein for left ventricular stimulation must be selected. The most constant and most accessible branches are the great cardiac vein and the mid cardiac vein. However, electrode insertion in these veins does not achieve the degree of synchrony we aim for. Various studies15,25 have shown that synchrony is best achieved by stimulating the left ventricular lateral wall because in complete left bundle branch block, this is the part of the left ventricle that suffers most from slowing of the electrical impulse. The coronary sinus branches that achieve greater synchrony are the lateral and posterolateral cardiac veins situated in the left ventricular lateral wall, although the existence of these varies from one patient to another. Once the electrode has been successfully located in the target vein, an appropriate threshold of capture is needed, as is the absence of diaphragmatic stimulation. If the stimulation threshold is greater than the diaphragmatic stimulation threshold, stimulation will prove painful and cannot be carried out. Both baseline and post-cardiac resynchronization electrocardiograms must be performed (Figures 3 and 4).
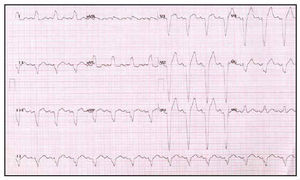
Fig. 3. Baseline electrocardiogram of a patient with heart failure refractory to treatment and complete left bundle branch block.
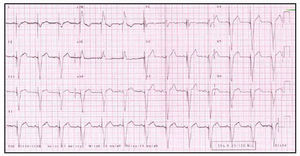
Fig. 4. Electrocardiogram of the same patient as in Figure 3 after implantation of the resynchronization system. Note the stimulation device, negative morphology of the QRS complex in the DI lead, and narrowing of the QRS complex.
Implanting physicians face a marked learning curve and this particularly limits first implants although the technique later becomes systematic. However, there remains a percentage of patients who cannot be implanted satisfactorily due to problems of coronary sinus access or the impossibility of optimal electrode placement. Implants entail risks we cannot ignore: dissection of the coronary sinus, tamponade and patients' poor tolerance of prolonged immobility. The long-term problems of electrode insertion in the coronary sinus, the need for future explantation (e.g. due to infection), and the accompanying complications, are as yet unknown.
CARDIAC RESYNCHRONIZATION: PROPOSED MECHANISM OF ACTION
Various mechanisms of action of cardiac resynchronization have been proposed. One of these is the increase in left ventricular filling time. Left bundle branch block delays ventricular stimulation but does not affect the atrium. Consequently, both passive ventricular filling and active filling by atrial contraction occur simultaneously leading to reductions in total transmitral blood flow and preload. Left ventricular stimulation facilitates earlier contraction and increases filling time. A second mechanism is the reduction in septal dyskinesia with a chronological disturbance caused by the septum moving away from the ventricle wall during systole. This paradoxical movement of the septal wall reduces the septum's contribution to left ventricular stroke volume. Another proposed mechanism is the reduction of mitral insufficiency. Pre-systolic mitral regurgitation is a frequent finding as papillary muscle activation is also delayed. Left ventricular stimulation produces early papillary muscle activation with a reduction in pre-systolic mitral regurgitation.
STUDIES OF THE ACUTE EFFECT IN PATIENTS UNDERGOING CARDIAC RESYNCHRONIZATION THERAPY
In 1994, Cazeau et al26 described the case of a patient with end-stage heart failure who made a spectacular improvement after left ventricular stimulation. Since then, during the 90s, many studies based on small numbers of patients have evaluated hemodynamic benefits found in patients undergoing acute cardiac resynchronization therapy. Unfortunately, these studies involved few patients and only evaluated short-term benefits. Their principal objectives were to determine acute hemodynamic parameters rather than long-term or overall mortality. Nor did they evaluate the repercussions of therapy on diastolic function.26 In most studies, dual-chamber pacemakers provided stimulation in an attempt to optimize atrioventricular interval. The patients enrolled presented severe heart failure (functional class III-IV) with depressed ejection fraction and complete left bundle branch block (QRS complex >150 ms). Results show that both patients undergoing biventricular cardiac resynchronization therapy and those with heart failure and delayed intraventricular conduction (complete left bundle branch block) receiving direct left ventricular stimulation presented a significant improvement in comparison with patients receiving no stimulation or direct right ventricular stimulation. In most cases, improvement took the form of increased left ventricular ejection fraction, increased cardiac index, reduced pulmonary capillary pressure or reduced myocardial oxygen uptake.25,27-31 Patients undergoing direct left ventricular stimulation presented the same or better acute hemodynamic parameters than those undergoing biventricular cardiac resynchronization therapy.27,29 Improvement in these patients was due to biventricular or left ventricular stimulation, rather than atrioventricular interval optimization, although this clearly led to an improvement in their hemodynamic condition. These studies did not reveal the effects of cardiac resynchronization therapy on diastolic function. It cannot be forgotten that up to 30%-40% of patients with heart failure, depending on the series, present diastolic dysfunction. Obviously, these patients do not obtain the same benefits from cardiac resynchronization therapy as those with predominantly systolic dysfunction.26
STUDIES OF THE CHRONIC EFFECT IN PATIENTS UNDERGOING CARDIAC RESYNCHRONIZATION THERAPY
To date, mid- and long-term studies demonstrate improvement in hemodynamic parameters in patients undergoing cardiac resynchronization therapy. Improvements are found in functional class, morbidity, and mortality, reduced left ventricular diameter, increased ejection fraction, increased oxygen uptake, lower rates of hospital admissions and improved 6-minute walking test performance25-38 (Table 1).
The Insync Italian Registry31 conducted a non-randomized analysis of 190 patients in functional class II-IV over 10±5 months. They found improvements in functional class, quality of life and 6-minute walking test performance. The PATH-CHF study32 was a simple blind, randomized, controlled, crossover study which compared single- and dual-chamber devices. The primary outcomes were exercise capacity measured by 6-minute walking test and symptoms. The study involved a 6 month follow-up with a 4 week period of no pacing. Results showed improvement in exercise capacity and functional class in patients undergoing biventricular therapy by comparison with those undergoing single-chamber stimulation. However, PATH-CHF included few patients, the researcher knew the treatment regime of each patient, and evaluation of symptoms and exercise capacity cannot be considered objective.
The MUSTIC33 and MUSTIC-AF34 studies were planned as two double blind, randomized, crossover, clinical trials, with 3 months of treatment with cardiac resynchronization therapy (coronary sinus inserted electrodes positioned at right ventricular apex and optimized atrioventricular interval). The MUSTIC-AF trial included patients with atrial fibrilla tion and slow cardiac frequency or atrioventricular node ablation. All patients had pacemakers. Patients were diagnosed with functional class III heart failure, ejection fraction ≤35% and wide QRS complex (≥150 ms in MUSTIC and ≥200 ms in MUSTIC-AF). The predicted minimum difference was 10%. Established outcomes were quality of life, oxygen uptake, 6-minute walking test performance and hospitalization. Initially 67 patients were enrolled in MUSTIC and 64 in MUSTIC-AF; later, a further 48 and 41 patients respectively, were recruited. Mean age was 64 years and most were men. In both cases a significant increase was found in oxygen uptake, 6-minute walking test performance and quality of life, as well as a lower rate of hospitalizations. These studies were the first randomized clinical trials to determine the value of cardiac resynchronization therapy. Their principal defect was that they offered no clear data on long-term reductions in mortality and morbidity.
In the MIRACLE study,35 266 patients were enrolled with a mean follow-up of 6 months. This was a double blind, prospective, parallel study. Only the implanting physician knew whether patients belonged to the control group or not. Patients were in functional class III with depressed ejection fraction (≤35%), wide QRS complex (≥130 ms) and increased end-diastolic diameter (>55 mm). Mean age was 64 years and 69% were men. Mean ejection fraction was 22%, end-diastolic volume was 70 mm, and mean QRS complex was 165 ms. Implantation success rate was 93%. The most significant results of MIRACLE were that patients undergoing cardiac resynchronization therapy improved in terms of symptoms, quality of life, exercise capacity and ejection fraction, with reduced left ventricular dimensions. However, the study design did not include morbidity and mortality.
Another study designed to evaluate long-term effects of cardiac resynchronization therapy was the double blind CONTAK-CD trial.36 All patients enrolled had indications for defibrillators and the devices implanted were capable of ventricular resynchronization with transvenous implantation of electrodes. Defibrillators were subcutaneous whereas in CONTAK-CHF implantation was by thoracotomy.37 The principal objective of CONTAK-CD was to determine the effect of cardiac resynchronization therapy on morbidity (hospitalization for heart failure, worsening of heart failure) and short-term mortality. Secondary objectives included exercise capacity and symptoms. Patients presented with functional class II-IV, depressed left ventricular ejection fraction (≤35%) and wide QRS complex (≥120 ms). Patients were in sinus rhythm and had indications for implantable automatic defibrillators. Of 501 patients recruited, 409 were randomized (mean age 66 years). Most patients (58%) were in functional class III, 33% were in class II and 9% in class IV. Mean left ventricular ejection fraction was 21% and mean QRS complex was 158 ms. Medical treatment was optimized to meet the tolerance levels of individual patients. Primary objective results showed no statistically significant difference as mortality was 5% and the study design predicted 15%. A predicted 30% worsening of heart failure was reduced in reality to 21%. In patients undergoing cardiac resynchronization therapy there were reductions in mortality (23%), hospitalizations (13%), and worsening of heart failure (26%). The reduction in left ventricular diameter, increase in peak oxygen uptake and improvement in functional class were all statistically significant. No significant differences were found in quality of life or 6-minute walking test performance.
A meta-analysis of 4 controlled clinical trials of cardiac resynchronization prior to 2002 (CONTAK-CD, InSync ICD, MIRACLE, and MUSTIC) confirmed a statistically significant 51% reduction in mortality for progressive heart failure (odds ratio [OR]=0.49; confidence interval [CI] 95%, 0.25-0.93). Similarly, there was a 23% reduction in total mortality in resynchronization patients, although this was not statistically significant.39
SELECTION OF PATIENTS FOR CARDIAC RESYNCHRONIZATION THERAPY
To ensure cardiac resynchronization therapy is cost-effective, it is vital we identify which patients benefit most (Table 2). Given that all studies and series report high rates of nonresponders, there is clearly no simple, conventional way of defining potential good responders. Current evidence questions the status of surface electrocardiograms and QRS duration as adequate predictors. Different criteria are used: e.g. quantifying left ventricular asynchrony and later correcting this as far as possible.
Analysis of published studies shows that patients who most benefit from cardiac resynchronization therapy present dilated cardiomyopathy with considerable systolic dysfunction (left ventricular ejection fraction <30%), increased left ventricular end-diastolic diameter (>60 mm), functional class III or IV heart failure and symptoms of heart failure despite optimal medical treatment.
However, in all clinical studies some 30% of patients do not improve with biventricular stimulation although there is no clear indication as to predictors of its failure or success. Although QRS duration has been the reference for indication and efficacy of resynchronization, reduced QRS duration does not predict response to therapy. There are several possible explanations. One is that the pattern of left bundle branch block, considered as that obtained from studying all of these patients, might really be heterogeneous, bringing together patterns of activation that may be totally different. Another possible predictoris the underlying heart disease, as response can differ in the presence of dilated cardiomyopathy or ischemic heart disease in the same way that ventricular activation and contraction can. It is also important to evaluate the effect of left bundle branch block, left ventricular stimulation, and mitral insufficiency. Authors have shown that improvement in the degree of mitral insufficiency is related to mid-term improvement in functional capacity.
Patients who receive resynchronization therapy generally have QRS complex duration of 120-200 ms. Yu et al40 found patients with a QRS complex of 120-150 ms benefited more than patients with QRS >150 ms. Nonresponders present a lesser degree of asynchrony in tissue Doppler imaging. Elsewhere, the same authors report patients with heart failure and narrow QRS complex do benefit from resynchronization. Approximately half of patients with heart failure and normal duration QRS complex present asymmetry.40
Several studies identify tissue echo-Doppler imaging as the best predictor of improved ventricular function and reduced ventricular volume as a consequence of resynchronization in patients with dilated cardiomyopathy. Others suggest tissue echo-Doppler can establish regional cardiac contraction delay and pre-select pacing sites.41,42 The as yet incomplete CARE-HF study employs inclusion criteria of echocardiographic asynchrony such as aortic ejection delay (>140 ms), interventricular aortic and pulmonary ejection delay >40 ms and left ventricular posterolateral wall activation delay.43 However, cardiac resynchronization has not demonstrated such effectiveness in functional class IV patients who require permanent inotropic support.44
The 2002 update of the guidelines of indications for pacemakers and defibrillators considered indication for cardiac resynchronization as class IIa in patients with idiopathic or ischemic dilated cardiomyopathy in functional class III-IV refractory to treatment, with prolonged QRS complex >130 ms, left ventricular diastolic diameter >55 mm and left ventricular ejection fraction <35% (level of evidence A).45
In a meta-analysis, Bradley et al39 include the latest multicenter studies of cardiac resynchronization and indicate that therapy reduces mortality for heart failure by 51% with respect to controls, with a relatively low rate of secondary effects.
Currently, level of evidence A has been established for the indication for biventricular stimulation therapy, which has been changed from IIA to I.
LEFT VENTRICULAR VERSUS BIVENTRICULAR STIMULATION
Studies of patients with acute illness have shown significant hemodynamic improvements after biventricular stimulation by comparison with isolated right ventricular stimulation. However, comparison of biventricular stimulation with isolated left ventricular stimulation produces different results and some studies show left ventricular stimulation is better. Hemodynamic data confirm that isolated left ventricular stimulation can improve parameters independently of right ventricular stimulation. Studies such as Auricchio et al46 suggest left ventricular stimulation is the key factor. So, isolated left ventricular stimulation patients with wide QRS complex may have the same results as those receiving biventricular stimulation.47 This questions the need for an additional right ventricular electrode implant. If a right ventricular electrode is used, the interventricular stimulation interval between electrodes must be quantified.
The BELIEVE study, which is incomplete at the time of writing, compares the effects of biventricular therapy with left ventricular stimulation in patients with a principal indication for automatic defibrillator implantation and heart failure associated with documented ventricular contraction asynchrony. This will be the first randomized study to compare biventricular stimulation with isolated left ventricular stimulation with a resynchronizing defibrillator.
ECHOCARDIOGRAPHIC OPTIMIZATION OF CARDIAC RESYNCHRONIZATION
In all patients, to obtain maximum benefit from treatment echocardiographic evaluation of ventricular asynchrony should be carried out individually both before and after biventricular stimulation device implantation in order to determine optimal parameters and ensure the best synchrony possible. Echocardiographic criteria of asynchrony include aortic pre-ejection delay >140 ms, mechanical interventricular delay (>40 ms) measured as the difference between the delay from the beginning of QRS complex to pulmonary ejection and the delay from the beginning of QRS complex to aortic ejection. Similarly, delayed activation of the posterolateral wall has been used as a marker.
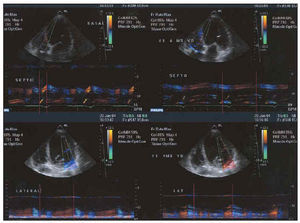
After electrode placement (in the right atrium if appropriate, right ventricle and coronary sinus lateral branches), echo-Doppler or, morerony. To improve the effects of biventricl must be optimized with the aid of mitral valve closure and atrial filling E and A waves,48,49 echo-Doppler imaging following Ritter et al,49 and interventricular interval or disturbances of stimulation between left and right ventricles. Presence of asynchrony (Figure 5 shows a study of asynchrony using tissue Doppler imaging), repercussions on systolic function (Figure 6), or presence of mitral insufficiency (Figure 7) must always be evaluated by echocardiography.
Fig. 5. M-mode tissue echo-Doppler analysis of degree of asynchrony. The 2 images on the left represent the time when peak systolic contraction occurs in the septum (above) and in the lateral face (below) with respect to the beginning of the QRS complex. Note the marked delay in the lateral face. The 2 images on the right represent the same times after biventricular stimulation. Synchronization of contraction times can be seen.
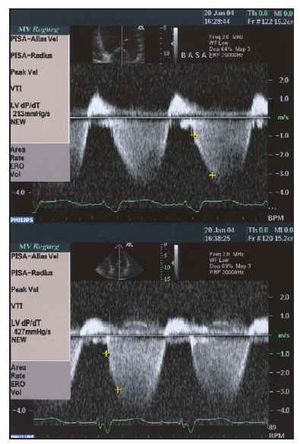
Fig. 6. Study of systolic function using dP/dT obtained from mitral insufficiency curve before and after resynchronization with biventricular pacemaker in the same patient as in Figure 1. Baseline dP/dT of 213 mm Hg/s improves to twice its value up to 427 mm Hg/s (below).
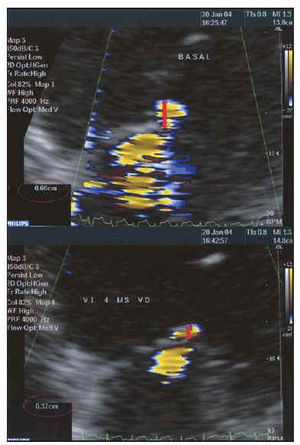
Fig. 7. Study of the severity of mitral insufficiency using the PISA method before (above) and after (below) resynchronization. Maintaining the same LN of 28.8 cm/s, the radius of the flow of proximal convergence is reduced by 0.66 to 0.37 cm, which implies that the regurgitated flow is reduced by 78.8 to 22.7 mL/s.
FOLLOW-UP
As patients undergoing cardiac resynchronization therapy have depressed ventricular function, functional class III-IV and ventricular conduction delay, it has been proposed that close follow-up should be carried out by a specialist team consisting of a nurse and a physician, both experts in heart failure management, and an electrophysiologist.
During checkups, a 12-lead electrocardiogram is used to analyze stimulation device function, optimal atrioventricular and interventricular delay, cardiac rhythm change and atrial fibrillation.
Patient education is fundamental and patients are taught to recognize signs and symptoms that indicate worsening of heart failure. Medical treatment is optimized. Three month checkups are recommended during the first year but frequency is reduced later. At 3 years, 3-monthly check-ups are reinstated as the battery approaches the end of its life.
As well as the clinical and hemodynamic parameters to evaluate response to biventricular stimulation therapy, biochemical parameters are starting to be used. Among these the most outstanding is monitoring natriuretic peptide (BNP) levels. Natriuretic peptides are secreted by the left ventricle and have been used to diagnose and follow-up patients with heart failure. Various studies show a fall in BNP values in response to cardiac resynchronization therapy.
PACEMAKER OR DEFIBRILLATOR FOR CARDIAC RESYNCHRONIZATION?
A substantial number of patients included in defibrillator studies present heart failure and/or ventricular dysfunction. This occurred in the AVID study,50 which included 1016 survivors of cardiac events caused by ventricular tachycardia or ventricular fibrillation. Approximately half the patients had heart failure and the majority had considerable ventricular systolic dysfunction. Patients with defibrillators presented a 50% lower risk of sudden death and non-cardiac death by comparison with patients receiving drug therapy. The MADIT II trials51 recruited patients with depressed ejection fraction (≤30%) and previous myocardial infarction. This study showed a 31% reduction in mortality by comparison with the conventional therapy group. A retrospective analysis by Stellbrink et al52 concluded that 7.3% of patients in functional class III with a defibrillator should be resynchronized, as should 12.5% of patients in functional classes II and III.
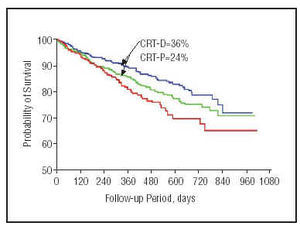
The COMPANION study53 has published preliminary results (Figure 8). At the time of writing, this is the widest-ranging study of patients with heart failure and a resynchronization device implant. Primary outcome was mortality and hospitalization for any cause in patients with heart failure. Secondary outcomes were hospitalization for cardiovascular cause, death from any cause, physical exercise performance and quality of life. The study recruited >1600 patients with medical treatment optimized to individual tolerance levels. Patients were divided into 3 groups: optimal drug treatment (20%); optimal drug treatment and a cardiac resynchronization therapy device (40%); optimal drug treatment and a device capable of cardiac resynchronization and defibrillation (40%). Preliminary results show a 19% reduction in the combined outcome of mortality from any cause and hospitalization in patients with heart failure and cardiac resynchronization pacemaker implants, and a 43% reduction in mortality from any cause for patients with heart failure and an implanted defibrillator with cardiac resynchronization capacity (P<.05).
Fig. 8. Kaplan-Meier curve showing COMPANION study results for 1-year mortality. The blue line shows the probability of patients with implanted defibrillators capable of resynchronization (significant 36% reduction in mortality); the green line represents patients with resynchronization associated with a pacemaker; and the red line, represents patients with optimal drug treatment.
On the other hand, the MIRACLE ICD study54 results show that the combination of a defibrillator with resynchronization improves quality of life, functional class and exercise duration in patients with malign ventricular arrhythmias and heart failure, without pro-arrhythmia and without compromising defibrillator efficiency. The BELIEVE study compares biventricular therapy with left ventricular stimulation in patients with a principal indication for an automatic defibrillator and heart failure associated with documented ventricular contraction asynchrony. When a defibrillator associated with resynchronization is used it is essential to check for adequate capture and avoid ventricular oversensing that might be caused by administration of inappropriate therapies.55
PRESENT AND FUTURE OF CARDIAC RESYNCHRONIZATION
Many issues remain to be resolved or confirmed with regard to cardiac resynchronization: does cardiac resynchronization therapy improve survival rates? Will it have the same effect in preventing sudden death as it has on mortality due to systolic dysfunction? What is the effect of resynchronization on long term ventricular remodeling? Will it have the same effect in asymptomatic patients or patients with few symptoms? What is the effect of cardiac resynchronization on the appearance of cardiac arrhythmias in patients with heart failure? How does biventricular stimulation affect patients who present diastolic dysfunction? Are results the same in patients with idiopathic or ischemic etiologies? Will patients in atrial fibrillation achieve the same benefits? What are the effects of resynchronization in patients with right bundle branch block?
Currently, different controlled studies are being conducted that will give us answers to most of these questions. The CARE-HF study42 has an open design and compares the effects of cardiac resynchronization therapy with a placebo. Its primary outcome is mortality from any cause and hospitalization for cardiovascular causes in patients with heart failure, systolic dysfunction and left ventricular asynchrony. The minimum follow-up will be 18 months. The PERFECT study is recruiting patients with heart failure or ventricular dysfunction who need cardiac stimulation devices in order to compare right ventricular stimulation with biventricular stimulation. Its principal objective is mortality and morbidity. Recruitment should take 2 years, with a 12 month follow-up.
Left ventricular stimulation may not only reverse the ventricular remodeling caused during bundle branch block, but it may also prevent this in specific patient groups, such as those receiving permanent right ventricular stimulation due to atrioventricular block. Several studies have shown that permanent right stimulation, even in patients with preserved left ventricular function, has a negative effect on cardiac function. The DAVID study18 published similar data which show that DDDR stimulation compared with VVI stimulation in patients with a defibrillator is associated with a higher combined rate of death or hospitalization or heart failure. Over 60% of DDDR patients receive continuous right stimulation. This issue is currently under study and data will be available in the future.
Not only has the effect of resynchronization been studied in patients with left bundle branch block. Its use has also been described in patients with right bundle branch block and ventricular dysfunction with improvement in hemodynamic parameters. These findings can also be interpreted in the context of diffuse illness of the bundle of His-Purkinje, with electrocardiographic disturbances both in the right branch and in the contralateral branch.
CONCLUSIONS
A high percentage of patients improve clinically after resynchronization device implantation. Consequently, cardiac resynchronization therapy is a valid alternative in many patients with ventricular systolic dysfunction associated with left bundle branch block. Equally, it is an option in patients who are not candidates for heart transplantation and as a bridging therapy prior to transplantation. Many issues remain unsolved. New studies currently in progress will help identify more clearly which patients benefit most from this treatment, and indicate how to optimize it.
Section Sponsored by Laboratorio Dr. Esteve
Correspondence: Dr. A. Hernández Madrid.
Unidad de Arritmias. Hospital Ramón y Cajal.
Ctra. Colmenar Viejo, km 9,100. 28034 Madrid. España.
E-mail: ahernandez.hrc@salud.madrid.org