We assessed the long-term hemodynamic performance of transcatheter heart valve (THV) by paired transthoracic echocardiography (TTE), and the incidence, characteristics and factors associated with THV structural valve degeneration (SVD).
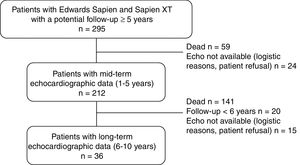
MethodsA total of 212 patients who underwent transcatheter aortic valve replacement and had a potential follow-up >5 years with at least 1 TTE ≥ 1-year postprocedure were included. All patients had a TTE at 1 to 5 years and 36 had another one at 6 to 10 years. SVD was defined as subclinical (increase >10mmHg in mean transvalvular gradient+decrease >0.3cm2 in valve area and/or new-onset mild or moderate aortic regurgitation) and clinically relevant (increase> 20mmHg in mean transvalvular gradient+decrease> 0.6cm2 in valve area and/or new-onset moderate-to-severe aortic regurgitation). Fifteen patients had a transesophageal echocardiography at the time of SVD diagnosis, and 85 an opportunistic computed tomography examination at 1 (0.5-2) years.
ResultsTransvalvular mean gradient increased and valve area decreased over time (P<.01). At 8 years of follow-up, SVD occurred in 30.2% of patients (clinically relevant: 9.3%). Transesophageal echocardiography revealed thickened and reduced-mobility leaflets in 80% and 73% of SVD cases, respectively. No baseline or procedural factors were associated with SVD. THV underexpansion (3.5%) or eccentricity (8.2%) had no impact on valve hemodynamics/SVD at follow-up.
ConclusionsA gradual THV hemodynamic deterioration occurred throughout a 10-year period, leading to SVD in ∼30% of patients (clinically relevant in < 10%). Leaflet morphology/mobility were frequently impaired in SVD cases, but THV geometry did not influence valve hemodynamics or SVD.
Keywords
Transcatheter aortic valve replacement (TAVR) is a first-line treatment for patients with severe symptomatic aortic stenosis at intermediate to high surgical-risk,1,2 and recent trials have provided the basis for its expansion to lower risk patients.3,4 Thus, whereas transcatheter heart valves (THV) longevity may have appeared trivial in the treatment of an initially elderly and high-risk population, it has currently become one of the most crucial aspects of TAVR.
Structural valve degeneration (SVD), common to both bioprosthetic surgical valves and THV, is the consequence of a multifaceted process represented mainly by organic tissue calcification and leaflet integrity disruption that ultimately lead to valve dysfunction.5–7 Good mid-term valve performance has been reported post-TAVR, but scarce data are available on long-term (> 5 years) THV durability. Furthermore, inconsistent results exist on long-term THV hemodynamic performance and the incidence of SVD.8–13 Among other explanations, this may be partially related to the nonpaired analyses of echocardiography data in most studies.8–12 Also, unlike surgical bioprostheses, THVs are subject to external forces leading to different degrees of underexpansion and/or eccentricity, which could potentially influence the leaflet-commissural stress and consequently, valve durability.14–17 However, no studies to date have evaluated the impact of THV frame circularity/underexpansion on valve durability and leaflet morphology findings in SVD cases. Thus, the objectives of this study were: a) to determine the long-term hemodynamic performance of THVs through paired transthoracic echocardiography (TTE), and b) to evaluate the incidence, characteristics, and factors associated with SVD.
METHODSA total of 295 consecutive patients with native severe symptomatic aortic stenosis underwent TAVR between 2007 and 2012 in our center. All patients received a balloon-expandable Edwards SAPIEN (n=170) or SAPIEN XT (n=125) valve (Edwards Lifesciences, United States). Patients had a clinical and echocardiography follow-up at 1 and 12 months, and yearly thereafter. Baseline, procedural and follow-up data were prospectively gathered in a dedicated database.
Echocardiographic assessmentTransthoracic echocardiography (TTE) studies performed by experienced echocardiographers before hospital discharge were used as a reference for comparison in order to assess the occurrence of SVD. Three distinct periods were predetermined for evaluating THV hemodynamics: hospital discharge; mid-term (1-5 years) and long-term (6-10 years) follow-up. The latest echocardiography from each period was used for the analyses. Mid-term echocardiographic assessment was obtained in 212 patients (90% of the population at risk) at a mean time of 3± 2 years, whereas 36 patients (70% of the population at risk) had long-term echocardiographic assessment at a mean time of 7± 1 years (up to 10 years post-TAVR) (figure 1).
Following current recommendations, qualitative and semiquantitative parameters were acquired during echocardiographic assessments.18,19 Mean transprosthetic gradient (MG) was obtained with the Bernoulli formula and the THV effective orifice area (EOA) was evaluated by the continuity equation. The left ventricular outflow tract diameter was measured immediately proximal to the THV stent frame.20 Differentiation between intraprosthetic (central) and paravalvular aortic regurgitation (AR) was performed by Color Doppler evaluation in several views, and its severity was assessed using a multiparameter integrative approach.
SVD was defined as either subclinical or clinically relevant as previously reported.20,21 Subclinical SVD was diagnosed in the presence of: a) an absolute increase in MG> 10 mmHg with a concomitant decrease in EOA> 0.3cm2 (and/or decrease in Doppler velocity index> 0.08), and/or new onset of at least mild intraprosthetic AR or increase by at least 1 grade of preexistent intraprosthetic valve regurgitation, with the resulting regurgitation grade inferior or equal to moderate; or b) change in morphology (ie, thickening, calcification, flail, pannus) and/or mobility (ie, reduced, avulsed) of THV leaflets. Clinically relevant SVD was defined as an increase in MG> 20 mmHg with a concomitant decrease in the EOA> 0.6 cm2 (and/or decrease in Doppler velocity index> 0.15), generating a severe aortic stenosis according to current guidelines; and/or new occurrence or increase of at least 1 grade of intraprosthetic regurgitation leading to moderate-to-severe AR.
Data on transesophageal echocardiography (TEE) examinations performed at the time of SVD diagnosis were collected. The presence of valvular thrombosis, leaflet calcification, morphology and mobility as well as AR severity and type were evaluated and recorded.
Multidetector computed tomographyIncidental multidetector computed tomography (MDCTs) were performed in 137 patients post-TAVR. Of these, the geometry and structural integrity of the THVs could be appropriately assessed in 85 examinations (40.1% of the 212 patients with at least 1 TTE at ≥ 1-year follow-up) performed at a median of 1 (0.5-2) year post-TAVR. An experienced radiologist, blinded to echocardiographic findings and the patient's clinical outcomes, reanalyzed all MDCTs targeting structural elements of the implanted THV such as fracture, circularity, and expansion. Although all MDCTs were electrocardiography (ECG)-gated, the examination protocol and the use of contrast media were determined by the radiologist responsible for their implementation.
The external edges of the THV were taken as reference to obtain its external diameter and area at 3 distinct cross-sectional levels (inflow, mid-stent, and outflow) during diastole at 75% of the R-R interval. After acquisition of such measurements, prosthesis expansion and circularity/eccentricity were assessed. THVs were circular when an eccentricity index, calculated as 1 – minimum THV external diameter/maximum THV external diameter, was <10%.17,22 THV underexpansion was defined as THV external area divided by nominal external area ≤ 90%.17,23
Statistical analysisContinuous variables were reported as mean± standard deviation or median [25th-75th interquartile range] as appropriate. Comparisons between numerical variables were performed by the Wilcoxon rank-sum or the Student t test. Categorical variables were reported as number (percent) and were compared using the chi-square or Fisher exact test. Changes in MG, EOA and intraprosthetic regurgitation over time were assessed with repeated measures analyses of variance. MG and EOA values were log transformed to stabilize variances. Reported P-values were based on this transformation. The incidence of SVD was assessed by death-competing risk analyses. Cox proportional-hazard models were applied to determine the factors associated with SVD. The relevant variables presenting a P-value <.10 in the univariable analyses were included in the multivariable model. Results were deemed significant when a 2-sided P-value <.05 was achieved. Statistical analyses were performed with the SAS statistical software version 9.4 (SAS Institute Inc., United States).
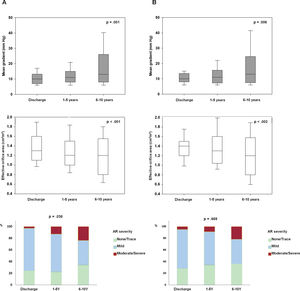
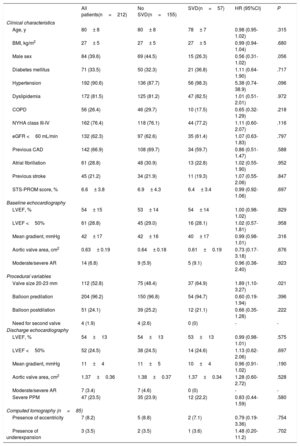
RESULTSTranscatheter heart valve hemodynamic performanceEchocardiographic data on THV hemodynamic changes over time, comprising the MG, EOA and intraprosthetic AR for the overall population are shown in figure 2A. A mild but significant increase in MG (Δ=1.94± 7.4 mmHg; P <.001), decrease in EOA (Δ=−0.09± 0.43cm2; P=.005) and a larger proportion of higher AR grades (P=.011) were observed from discharge to mid-term follow-up. A further increase in MG (Δ=6.0± 11.9 mmHg; P=.004) along with a decrease in EOA (Δ=−0.19± 0.44cm2; P=.02), and an increase in the proportion of higher AR grades (P=.038) were also observed from mid- to long-term follow-up.
Changes in transcatheter valve hemodynamics over time. A: unpaired echocardiographic data of patients with at least 1 transthoracic echocardiography 1 year after TAVR (n=212). P <.001 for changes in both mean gradient and effective orifice area and P=.038 for the increase in the proportion of higher grades of intraprosthetic regurgitation from discharge to 6 to 10 years of follow-up. B: paired echocardiographic data of patients with transthoracic echocardiography assessment at hospital discharge, mid-term (1-5 years) and long-term (6-10 years) follow-up (n=36). P=.006, P=.002 and P=.669 for changes in mean gradient, effective orifice area and for the increase in the proportion of higher grades of intraprosthetic regurgitation, respectively. AR, aortic regurgitation.
Paired echocardiographic data from 36 patients obtained at the 3 prespecified time periods revealed a significant increase in MG and a decrease in EOA (Δ=5.9± 11.8, P=.006; Δ=−0.19± 0.44cm2, P=.002, respectively), although no significant change in AR severity was observed (P=.669) throughout the follow-up (figure 2B).
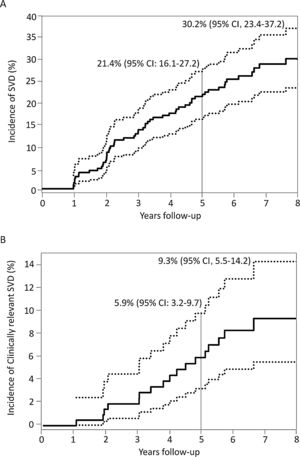
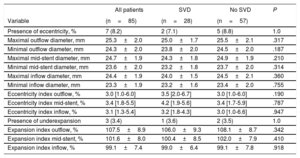
Incidence, characteristics and factors associated with structural valve degenerationThe main baseline, procedural, discharge echocardiography and post-TAVR MDCT data of patients who had at least 1 echocardiographic assessment at ≥ 1-year after TAVR are shown in table 1 according to SVD status. SVD occurred in 57 patients (26.9%) and was subclinical and clinically relevant in 40 (18.9%; 70.2% of the SVD cohort) and 17 patients (8.0%; 29.8% of the SVD cohort), respectively. The overall incidence of SVD at 8 years of follow-up was 30.2%; 95%CI, 23.4%-37.2%, where 21.9%; 95%CI, 15.8%-28.6% and 9.3%; 95%CI, 5.5%-14.2% were subclinical and clinically relevant, respectively (figure 3). The mean time for the diagnosis of any SVD was 3± 2 years, hence the majority (48 patients, 84%) was diagnosed at a mid-term follow-up; 34 (60%) and 14 (24%) being subclinical and clinically relevant SVD, respectively. At long-term follow-up, another 9 (16%) SVD cases occurred, of which 6 (11%) were subclinical and 3 (5%) were clinically relevant (figure 1 of the supplementary data).
Baseline, procedural, discharge echocardiography and post-TAVR MDCT data according to the presence of structural valve degeneration
| All patients(n=212) | No SVD(n=155) | SVD(n=57) | HR (95%CI) | P | |
|---|---|---|---|---|---|
| Clinical characteristics | |||||
| Age, y | 80± 8 | 80± 8 | 78± 7 | 0.98 (0.95-1.02) | .315 |
| BMI, kg/m2 | 27± 5 | 27± 5 | 27± 5 | 0.99 (0.94-1.04) | .680 |
| Male sex | 84 (39.6) | 69 (44.5) | 15 (26.3) | 0.56 (0.31-1.02) | .056 |
| Diabetes mellitus | 71 (33.5) | 50 (32.3) | 21 (36.8) | 1.11 (0.64-1.90) | .717 |
| Hypertension | 192 (90.6) | 136 (87.7) | 56 (98.3) | 5.38 (0.74-38.9) | .096 |
| Dyslipidemia | 172 (81.5) | 125 (81.2) | 47 (82.5) | 1.01 (0.51-2.01) | .972 |
| COPD | 56 (26.4) | 46 (29.7) | 10 (17.5) | 0.65 (0.32-1.29) | .218 |
| NYHA class III-IV | 162 (76.4) | 118 (76.1) | 44 (77.2) | 1.11 (0.60-2.07) | .116 |
| eGFR <60 mL/min | 132 (62.3) | 97 (62.6) | 35 (61.4) | 1.07 (0.63-1.83) | .797 |
| Previous CAD | 142 (66.9) | 108 (69.7) | 34 (59.7) | 0.86 (0.51-1.47) | .588 |
| Atrial fibrillation | 61 (28.8) | 48 (30.9) | 13 (22.8) | 1.02 (0.55-1.90) | .952 |
| Previous stroke | 45 (21.2) | 34 (21.9) | 11 (19.3) | 1.07 (0.55-2.06) | .847 |
| STS-PROM score, % | 6.6± 3.8 | 6.9± 4.3 | 6.4± 3.4 | 0.99 (0.92-1.06) | .697 |
| Baseline echocardiography | |||||
| LVEF, % | 54± 15 | 53± 14 | 54± 14 | 1.00 (0.98-1.02) | .829 |
| LVEF <50% | 61 (28.8) | 45 (29.0) | 16 (28.1) | 1.02 (0.57-1.81) | .958 |
| Mean gradient, mmHg | 42± 17 | 42± 16 | 40± 17 | 0.99 (0.98-1.01) | .316 |
| Aortic valve area, cm2 | 0.63± 0.19 | 0.64± 0.18 | 0.61±0.19 | 0.73 (0.17-3.18) | .676 |
| Moderate/severe AR | 14 (6.8) | 9 (5.9) | 5 (9.1) | 0.96 (0.38-2.40) | .923 |
| Procedural variables | |||||
| Valve size 20-23 mm | 112 (52.8) | 75 (48.4) | 37 (64.9) | 1.89 (1.10-3.27) | .021 |
| Balloon predilation | 204 (96.2) | 150 (96.8) | 54 (94.7) | 0.60 (0.19-1.94) | .396 |
| Balloon postdilation | 51 (24.1) | 39 (25.2) | 12 (21.1) | 0.66 (0.35-1.28) | .222 |
| Need for second valve | 4 (1.9) | 4 (2.6) | 0 (0) | - | - |
| Discharge echocardiography | |||||
| LVEF, % | 54±13 | 54±13 | 53±13 | 0.99 (0.98-1.01) | .575 |
| LVEF <50% | 52 (24.5) | 38 (24.5) | 14 (24.6) | 1.13 (0.62-2.06) | .697 |
| Mean gradient, mmHg | 11±4 | 11±5 | 10±4 | 0.96 (0.91-1.02) | .190 |
| Aortic valve area, cm2 | 1.37±0.36 | 1.38±0.37 | 1.37±0.34 | 1.28 (0.60-2.72) | .528 |
| Moderate/severe AR | 7 (3.4) | 7 (4.6) | 0 (0) | - | - |
| Severe PPM | 47 (23.5) | 35 (23.9) | 12 (22.2) | 0.83 (0.44-1.59) | .580 |
| Computed tomography (n=85) | |||||
| Presence of eccentricity | 7 (8.2) | 5 (8.8) | 2 (7.1) | 0.79 (0.19-3.36) | .754 |
| Presence of underexpansion | 3 (3.5) | 2 (3.5) | 1 (3.6) | 1.48 (0.20-11.2) | .702 |
95%CI, 95% confidence interval; AR, aortic regurgitation; BMI, body mass index; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; HR, hazard ratio; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; PPM, prosthesis-patient mismatch; STS-PROM, Society of Thoracic Surgeons-Predicted Risk of Mortality; SVD, structural valve degeneration.
Unless otherwise indicated, values are expresses as No. (%), or mean±standard deviation.
The development or increase in intraprosthetic AR was the most frequent cause of SVD in both the subclinical (27 patients, 68%) and the clinically relevant (12 patients, 71%) cohorts. While isolated THV stenosis was the second cause of SVD in the subclinical cohort (10 patients, 25%), it was the least frequent cause in clinically relevant SVD patients (1 patient, 6%). Mixed valve dysfunction, with THV stenosis and intraprosthetic AR criteria, was the primary cause of SVD in 3 (7%) and 4 (23%) patients in the subclinical and clinically relevant SVD cohorts, respectively.
There were 16 patients with SVD among the 36 patients with long-term echocardiography data. This specific subgroup showed a significant increase in MG (Δ=13.2± 14.5 mmHg; P <.001) and in the proportion of higher grades of intraprosthetic AR (P=.045) with a decrease in EOA (Δ=−0.32± 0.55cm2; P <.001) over time, regardless of SVD type (figure 2 of the supplementary data).
SVD worsened over time, since 3 (7.5%) subclinical SVD patients developed clinically relevant SVD, and mixed valve dysfunction was eventually observed in 2 (20%) and 4 (14.8%) patients initially diagnosed with isolated THV stenosis and isolated intraprosthetic AR, respectively.
Aortic valve reintervention was performed in 12 (5.66%) patients overall. The reasons for reintervention were subclinical SVD in 4 (33%) patients, clinically relevant SVD in 5 patients (42%), development of nonstructural valve deterioration in the form of severe paravalvular regurgitation in 2 (17%) patients and 1 (8%) patient was reintervened in another country, due to unknown causes. All but one reintervened SVD patient underwent a TAVR-in-TAVR procedure. All cases with nonstructural valve deterioration were treated by surgical aortic valve replacement (SAVR).
Although smaller THVs (P=.021) were more frequent in the SVD cohort (table 1), no individual factor was independently associated with SVD (multivariable analysis) (table 1 of the supplementary data).
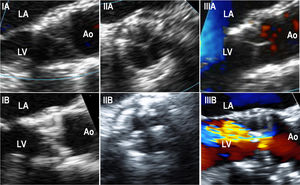
Transesophageal echocardiography dataFifteen patients underwent a TEE assessment at the time of SVD diagnosis (mean: 5± 1 years post-TAVR) (table 2 of the supplementary data). In this cohort, SVD was most frequently secondary to the appearance/increase of intraprosthetic regurgitation (8 patients, 53.3%), followed by THV stenosis (5 patients, 33.3%) and mixed THV degeneration (2 patients, 13.3%). No THV thrombosis was observed. TEE accurately determined the grade and type of AR while displaying thickened/calcified leaflets and abnormal leaflet mobility in 12 (80%) and 11 (73%) patients, respectively (figure 4).
Transesophageal echocardiography images performed in structural valve degeneration patients (IA). Intraprocedural long-axis view of a SAPIEN XT showing thin THV leaflets (IB). Long-axis view of a SAPIEN XT performed 5.5 years after the implantation on a patient with THV due to stenosis, showing thickened and calcified leaflets (IIA). Intra-procedural short-axis view of a SAPIEN XT showing thin THV leaflets (IIB). Short-axis view of a SAPIEN XT performed 5.5 years after the THV implantation on a patient with THV due to stenosis, showing thickened and calcified leaflets (IIIA). Color-Doppler intraprocedural long-axis view of a SAPIEN XT showing thin THV leaflets and no residual aortic regurgitation (IIIB). Color-Doppler long-axis view of a SAPIEN XT performed 5.3 years after THV implantation in a patient with THV due to the development of severe intraprosthetic aortic regurgitation, although thickened and calcified THV leaflets were also observed. Ao, aorta; LA, left atrium; LV, left ventricle; THV, transcatheter heart valve.
The clinical and procedural characteristics of the 85 patients with analyzable MDCT examinations at follow-up are shown in table 3 of the supplementary data. At a median of 1 (0.5-2) year post-TAVR, no stent frame fractures were observed among the 85 analyzable MDCT studies. Overall, 7 (8.2%) THVs were eccentric and 3 (3.4%) were underexpanded. Although a significantly smaller expansion was observed at the inflow when compared with the outflow level (P <.001), THV circularity remained similar at all levels (P=.993).
Data obtained from the discharge and last available TTE showed no impact of THV eccentricity and/or underexpansion on echocardiographic parameters over time (MG, P=.947; EOA, P=.594; AR, P=.119) (figure 5). A total of 28 SVD patients (49.1% of the SVD cohort) were among the MDCT cohort, and no differences in MDCT measurements and THV eccentricity and/or underexpansion were observed between SVD and non-SVD patients (P=ns for all) (table 2).
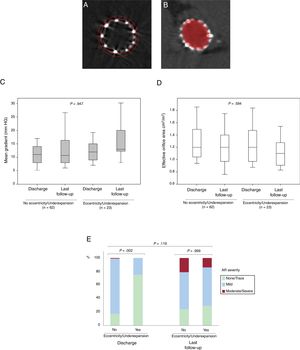
Changes in valve hemodynamics from discharge to the last follow-up echocardiogram for the cohort with performance of multidetector computed tomography after TAVR according to THV eccentricity and expansion. A: MDCT image of an underexpanded 26mm SAPIEN valve; dotted line delineates the nominal THV area whereas the solid line delineates the achieved THV area. B: MDCT image of an eccentric 23-mm SAPIEN valve; elliptic figure demonstrates the THV eccentricity. C,D: mean aortic gradient and effective orifice area. P=.947 and P=.594 for the difference between the absence and the presence of eccentricity/underexpansion regarding transprosthetic mean gradient and effective orifice area at discharge and at the last available echocardiogram at follow-up, respectively. E: intraprosthetic valve regurgitation. P=.119 for changes in aortic severity proportions from discharge to last available echocardiogram at follow-up. AR, aortic regurgitation; MDCT, multidetector computed tomography; TAVR, transcatheter aortic valve replacement; THV: transcatheter heart valve.
Multidetector computed tomography data according to the presence of structural valve degeneration (n=85)
| All patients | SVD | No SVD | P | |
|---|---|---|---|---|
| Variable | (n=85) | (n=28) | (n=57) | |
| Presence of eccentricity, % | 7 (8.2) | 2 (7.1) | 5 (8.8) | 1.0 |
| Maximal outflow diameter, mm | 25.3±2.0 | 25.0±1.7 | 25.5±2.1 | .317 |
| Minimal outflow diameter, mm | 24.3±2.0 | 23.8±2.0 | 24.5±2.0 | .187 |
| Maximal mid-stent diameter, mm | 24.7±1.9 | 24.3±1.8 | 24.9±1.9 | .210 |
| Minimal mid-stent diameter, mm | 23.6±2.0 | 23.2±1.8 | 23.7±2.0 | .314 |
| Maximal inflow diameter, mm | 24.4±1.9 | 24.0±1.5 | 24.5±2.1 | .360 |
| Minimal inflow diameter, mm | 23.3±1.9 | 23.2±1.6 | 23.4±2.0 | .755 |
| Eccentricity index outflow, % | 3.0 [1.0-6.0] | 3.5 [2.0-6.7] | 3.0 [1.0-6.0] | .190 |
| Eccentricity index mid-stent, % | 3.4 [1.8-5.5] | 4.2 [1.9-5.6] | 3.4 [1.7-5.9] | .787 |
| Eccentricity index inflow, % | 3.1 [1.3-5.4] | 3.2 [1.8-4.3] | 3.0 [1.0-6.6] | .947 |
| Presence of underexpansion | 3 (3.4) | 1 (3.6) | 2 (3.5) | 1.0 |
| Expansion index outflow, % | 107.5±8.9 | 106.0±9.3 | 108.1±8.7 | .342 |
| Expansion index mid-stent, % | 101.6±8.0 | 100.4±8.5 | 102.0±7.9 | .410 |
| Expansion index inflow, % | 99.1±7.4 | 99.0±6.4 | 99.1±7.8 | .918 |
SVD, structural valve degeneration.
Data are expressed as No. (%), mean±standard deviation, or median [interquartile range].
The main findings of this study including balloon-expandable THV recipients are as follows: a modest but significant decrease in EOA and increase in MG were observed over a 10-year follow-up period; b) SVD occurred in about one fourth of patients (subclinical and clinically relevant in two thirds and one third of SVD patients, respectively); c) most SVD patients assessed by TEE exhibited thickened and reduced-mobility leaflets, and none had signs of THV thrombosis; and d) MDCT showed no stent frame fracture, a low incidence of THV eccentricity and/or underexpansion, and no association between THV circularity/expansion and SVD was observed.
Currently, inconsistent data on THV hemodynamics are available, with some reports revealing steady EOAs and MG up to 5 years of follow-up,24–29 while others showed a significant decrease in EOAs and a trend toward MG increase.7,30 Recently, studies with longer follow-up demonstrated stable THV hemodynamics beyond 5 years.10–12 However, none of these reported paired echocardiography data. Interestingly, a recent publication with a paired comparison between discharge and the last available TTEs, showed lower peak transprosthetic gradients and a reduction in AR severity at long-term follow-up.13 However, these findings were mainly driven by self-expandable THVs (CoreValve system in about two thirds of patients), since no change in valve hemodynamics was observed among the balloon-expandable valve recipients.13 Conversely, our paired TTE analyses performed with data from 3 different time points showed a gradual and significant increase in MG with a decrease in EOA over time. Interestingly, this analysis revealed AR worsening exclusively among the SVD cohort.
Whereas SVD occurs in <5% of TAVR patients up to 5 years,7,24–30 rates ranging from 0% to 50% have been reported beyond the 5-year landmark.9–13,31 Notably, SVD definitions have diverged among previous reports. Nevertheless, long-term incidences of SVD still varied from 0% to 15%, on application of the criteria proposed by the European Association of Percutaneous Cardiovascular Interventions EACTS-EAPCI32 at similar follow-up periods.9–13 A potential shortcoming of this definition is the acceptance of absolute values of MG ≥ 20 mmHg at any echocardiography during follow-up as diagnostic of SVD. This may lead to substantial overestimation of the SVD incidence, since it may categorize severe prosthesis-patient mismatch cases as SVD. The definition used in our study does not include absolute values of MG as diagnostic of SVD and mandates the presence of valve hemodynamic deterioration during follow-up to confirm the presence of SVD. Recent data from the NOTION trial revealed rates of SVD as 24% in SAVR and 4.8% in TAVR according to the European Association for Cardio-Thoracic Surgery-European Association of Percutaneous Cardiovascular Interventions (EACTS-EACPI) definitions, but 2-fold lower (14.1% and 2.1%, respectively), when considering the hemodynamic deterioration throughout follow-up.10 Finally, accurate Doppler measurements are highly important for the diagnosis of SVD, and the variability in such measurements at different time points following TAVR may also impact the rates of SVD reported in different studies.
Understandably, the primary cause of SVD varies according to the diagnostic criteria applied. In a recent review, Foroutan et al.6 found that post-TAVR SVD was mainly due to THV stenosis (58%), with regurgitation (39%) and mixed etiology (3%) being less frequent, whereas in our study, most SVD cases had intraprosthetic AR as the primary cause. Although SVD is known to be a gradual process, our study is the first to demonstrate the progression of THV degeneration over a long-term follow-up, as perceived by the changes in severity (7.5% of subclinical SVD progressed to clinically relevant SVD) and in SVD criteria (20% and 15% of SVD due to THV stenosis and intraprosthetic AR, respectively, developed mixed THV dysfunction).
In the field of SAVR, SVD has traditionally been equivalent to valve reintervention.21,33 Nevertheless, applying the same SVD definitions as in our study, Rodriguez-Gabella et al.21 revealed rates of subclinical and clinically relevant SVD of 30.1% and 6.6%, respectively, in a SAVR cohort with a median follow-up of 10 years. In another SAVR series, Salaun et al.34 reported a 30.9% rate of overall SVD at a median follow-up of 10 years. These results are therefore similar to those observed in our TAVR cohort.
The crimping process, the ever-thinning leaflets, the higher leaflets-frame interaction due to asymmetric and/or incomplete THV expansion, have all been considered potential factors for higher SVD development in TAVR when compared with surgical bioprosthesis.5,14–16,22 Nevertheless, fewer severe prosthesis-patient mismatches and lower residual gradients in THV recipients might overcome such detrimental aspects, thus explaining the significantly higher rates of SVD after SAVR when compared with TAVR observed in the NOTION trial, even when applying a more rigorous SVD definition.10
Similar to a previous MDCT study of balloon-expandable THVs,17 no THV stent frame fractures were observed in our study. Moreover, the THV eccentricity rate (8%) was within the previously reported range (4%-14%).17,35 Delgado et al.35 showed an association between higher eccentricity rates, severe aortic valve calcification and postprocedure incidence of moderate AR observed by TTE assessments at 1-month post-TAVR. On the other hand, Willson et al.17 reported a lack of association between THV circularity and valve hemodynamics at 1-year follow-up. Similarly, our study reports for the first time the lack of association between THV eccentricity/underexpansion and poorer hemodynamic performance and/or SVD development at long-term follow-up. Furthermore, although our rate of underexpanded THVs (3.4%) was lower than the 8% reported by Wilson et al.,17 both studies revealed significantly smaller expansion indexes at THV inflow when compared with the outflow level. The restriction force of the aortic annulus, especially in the presence of an excessive THV oversizing, and the lack of biological tissue resistance at the outflow level likely explain the difference in expansion at different THV levels.
Echocardiographic evaluation of TAVR recipients is currently recommended at hospital discharge, 6 and 12 months and yearly thereafter.18,19 Although TTE should be the first modality performed, TEE is strongly advocated in the presence of abnormal findings on TTE.36 Whereas leaflet thickening and calcification are often observed after longer time periods, THV thrombosis has been traditionally described as an early event.7,37 Nevertheless, histological analyses of explanted THVs revealed that although leaflet fibrosis and calcification appeared to be associated with the length of follow-up, valvular thrombosis occurred regardless of the time period.38 Hence, detailed imaging of SVD patients is crucial in determining the cause of valvular deterioration. Our study is the first to provide TEE data of SVD patients obtained at long-term follow-up. Overall, valvular thrombosis was accurately ruled out as the cause of SVD and thickened and reduced-mobility leaflets were present in the vast majority of cases. Lastly, TEE accurately determined the grade and type of AR, thus helping to determine the most appropriate treatment strategy.
LimitationsThe high-risk profile and advanced age of the study population translated into a high mortality rate at follow-up, which negatively impacted the number of patients at risk for SVD development. While the performance of yearly echocardiographic examinations post-TAVR was prespecified, echocardiography data at follow-up was incomplete. However, paired echocardiographic analysis was available in up to 90% and 70% of patients at risk at 1 to 5 and> 5-year follow-up, which is similar or higher than the rate reported in prior studies in the field. Core laboratory analysis was not available in the present study. In addition, MDCT studies were opportunistic and without contrast injection, which precluded a more accurate evaluation (vs TEE) of valve thrombosis. Furthermore, TEE and MDCT studies were not systematically performed, and a potential selection bias might have influenced the results related to these imaging modalities. However, this was partially compensated by the fact that no major clinical differences were observed between those patients with and without MDCT studies at follow-up (table 3 of the supplementary data). Finally, no data were available on vasodilator medication at the time of echocardiography examination at follow-up.
CONCLUSIONSA mild but significant gradual deterioration of THV hemodynamics occurred throughout a 10-year follow-up period. This translated into overall and clinically relevant SVD rates at 8-year follow-up of about 30% and 9%, respectively, which appears comparable to those reported in surgical series. Importantly, THV stent frame geometry as determined by MDCT failed to impact valve hemodynamic changes over time. Future studies with a larger number of patients and even longer follow-up periods are needed to further elucidate the incidence and factors associated with SVD.
- –
TAVR is an established treatment for severe symptomatic aortic stenosis patients at intermediate to high surgical-risk.
- –
Its expansion to lower risk patients has rendered the long-term durability of THV crucial.
- –
Currently, scarce and controversial information on the long-term hemodynamics of THVs is available, and no data exist on (i) the long-term impact of THV geometry on valve durability and (ii) leaflet morphology/mobility in SVD cases.
- –
This study of 212 THV recipients revealed a significant increase in mean gradient and a decrease in THV area over time.
- –
At 8 years of follow-up, despite mild hemodynamic deterioration, less than a third of patients developed SVD (clinically relevant in < 10%); TEE revealed leaflet thickening and reduced-mobility in most SVD THVs evaluated and MDCT showed no impact of THV geometry on valve hemodynamic changes and development of SVD over time.
P. Pibarot has Core Lab contracts with Edwards Lifesciences for which he receives no direct compensation. J. Rodés-Cabau has received institutional research grants from Edwards Lifesciences and Medtronic. All other authors have reported that they have no relationships relevant to the contents of this article to disclose.
J. Rodés-Cabau holds the Research Chair “Fondation Famille Jacques Larivière” for the Development of Structural Heart Disease Interventions.
Supplementary data associated with this article can be found in the online version available at https://doi.org/10.1016/j.rec.2020.02.002