When fibrinolysis fails in patients with ST elevation myocardial infarction, they are referred for a rescue percutaneous coronary intervention (PCI). However, there is still no evidence of how much myocardium potentially at risk we can actually salvage after rescue PCI.
MethodsFifty consecutive patients. Cardiac magnetic resonance was performed within 6 days. Myocardial necrosis was defined by the extent of abnormal late enhancement, myocardium at risk by extent of edema, and the amount of salvaged myocardium by the difference between myocardium at risk and myocardial necrosis. Finally, myocardial salvage index (MSI) resulted from the fraction (area-at-risk minus infarct-size)/area-at-risk.
ResultsThe mean time elapsed between pain onset and fibrinolitic agent administration was 176±113min; time lysis-rescue=PCI 209±122min; time pain onset-PCI=390±152min. The area at risk was 37%±13% and infarct size 34.5%±13%. Salvaged myocardium was 3%±4% and MSI 9±8. Salvaged myocardium and MSI were similar between patients with the artery open on arrival at the catheterization lab (Thrombolysis in Myocardial Infarction [TIMI] 3) and those with TIMI flow ≤2 (3.3%±3.6% and 8.2±6.9 in TIMI 0-2 vs 3.0%±3.7% and 10.8±10.9 in TIMI 3; P=.80 and 0.31, respectively). No significant difference was observed between patients who went through rescue PCI within a shorter time and those with longer delay times.
ConclusionsThe myocardial salvage after rescue PCI quantified by cardiac magnetic resonance is very small. The long delay times between pain onset and the opening of the infarct-related artery with PCI are most probably the reason for such a minimal effect of rescue PCI.
Keywords
The best therapy in patients with ST elevation myocardial infarction (STEMI) achieves fast, adequate, and sustained reperfusion of the artery related to the episode. Although primary percutaneous coronary intervention (PCI) has proved superior to fibrinolysis,1, 2 the latter is still the most frequently employed option in about 50% of patients, mainly due to the logistic difficulties of performing primary PCI. In 30% to 50% of cases, fibrinolysis fails to restore an adequate flow in the vessel (Thrombolysis in Myocardial Infarction [TIMI] 3 flow),3 and those patients present a higher–both early and late–mortality rate compared to patients achieving an adequate flow.4 In those cases, rescue angioplasty is often used to restore a TIMI 3 flow. Nevertheless, the benefits in terms of mortality rate reduction achieved with this procedure are being thoroughly discussed at present after the results of the clinical trials published so far, namely: MERLIN (Middlesbrough early revascularization to limit infarction)5 and REACT (Rescue angioplasty after failed thrombolytic therapy for acute myocardial infarction),6 the level of recommendation currently used in European and American guides being IIa.7, 8
A number of clinical trials published recently have used different image techniques to successfully assess the quantity of myocardium at risk that we can salvage in an acute myocardial infarction (AMI) after reperfusion.9, 10 However, it remains unclear how much myocardium potentially at risk we can actually salvage after performing rescue angioplasty.
Cardiac magnetic resonance (CMR) has emerged as the ideal technique for the integral study of patients with ischemic cardiomyopathy, as it permits us not only to evaluate myocardial function but also to determine AMI extension within the same study.11
According to our hypothesis, the quantity of salvaged myocardium after failed fibrinolysis and rescue angioplasty in patients with STEMI–characterized by CMR–is rather low, basically due to the delay in the different execution times.
The present paper has as its primary objective to quantify the salvaged myocardium after rescue angioplasty following failed fibrinolysis in patients with AMI using CMR, its secondary objective being to evaluate the link between the variation regarding delay times in angioplasty and the amount of salvaged myocardium.
METHODS Study PopulationThis prospective trial was conducted at a single tertiary care center between June 2008 and February 2009. We included 63 consecutive patients who underwent rescue PCI at our catheterization lab. These patients had been referred for rescue PCI after the failure of fibrinolysis at 7 “secondary” hospitals–centers where neither catheterization laboratories nor a team of interventional cardiologists on call were available, which made fibrinolysis the elective method for STEMI treatment. STEMI was defined by the presence of ischemic chest pain lasting longer than 30min, unrelieved by sublingual nitrate and associated with typical ST-segment elevation on the 12-lead electrocardiogram (ECG) (at least 2mm of ST-segment elevation in 2 or more contiguous chest leads and at least 1mm in 2 or more contiguous limb leads). All patients received a weight-adjusted dose of fibrin-specific agent (tenecteplase); acetylsalycilic acid, 300mg orally; an oral loading dose of clopidogrel, 300mg; and enoxaparin intravenous bolus followed by a first subcutaneous dose 15min later. Failure to respond to fibrinolytic therapy was defined by a second 12-lead ECG obtained 90min after starting fibrinolytic therapy, which showed ST-segment elevation failure in the worst lead to have resolved by 50% with respect to the pretreatment ECG (ST-segment measured 80mg after the J point). At that moment, the interventional cardiologist on call was contacted and the patient was transferred to our center so that rescue PCI could be performed.
Exclusion CriteriaPatients presenting clinical unstability after rescue PCI (severe arrhythmia or heart failure) or those with known contraindications for CMR (claustrophobia, pacemakers, or implantable defibrillator devices) were finally excluded. Furthermore, patients with a previous infarction–regardless of its localization, in the same place or elsewhere–were also excluded in order to avoid confusion when calculating the quantity of salvaged myocardium.
The study protocol was approved by the local ethics committee, and all patients gave their written informed consent once the purpose of this test was explained to them.
Rescue AngioplastyGlycoprotein IIb/IIIa inhibitor Abciximab was given intravenous as a bolus of 0.25mg/kg bolus, 0.125mg/kg/min infusion (maximum 10mg/min for 12h) at the operator's discretion in the catheterization lab. The responsibility for the choice of radial or femoral access, catheter diameter (5-7 F), stent type (bare metal stent or drug eluting stent) as well as thrombectomy also fell upon the operator.
We recorded patients’ clinical, demographic, and angiographic characteristics on admission and also analyzed the time elapsed from the first symptoms to the start of fibrinolytic therapy, time between the unsuccessful thrombolysis and the patient's arrival at the catheterization lab and, finally, the global time elapsed between symptom onset and balloon inflation. The angiography projections used were those allowing optimal TIMI flow evaluation in the infarct-related artery (grade 0=no antegrade flows beyond the occlusion point; grade 1=contrast material passes beyond the obstruction area but fails to opacify the entire bed distal to the obstruction; grade 2=contrast material passes across the obstruction and opacifies the coronary bed distal to the obstruction but more slowly than the normal flow; grade 3=normal flow).3 Angiographic analysis included pre- and post-PCI flow in the guilty vessel as the presence of visible thrombus. The amount of vessel disease (arteries with at least one lesion >50%) along with the ejection fraction were also examined. Angiographic nonreflow was defined as the absence of adequate reflow (TIMI 3 flow) after PCI without angiographic evidence of mechanical vessel obstruction.
These data were reviewed off-line by a single expert investigator blinded both to clinical information and to results. An ECG was performed 30min after procedure. It was analyzed by a single cardiologist also blinded to clinical data, ST resolution being accepted if it fell by more than 50% with respect to the initial ECG.
Cardiac Magnetic ResonanceCMR was performed within a maximum of 6 days (range 2-6) in an effort to ensure that CMR findings were due to acute changes.
Image AcquisitionA 1.5. T scanner (Intera CV, Philips Medical Systems, Best, the Netherlands) and a 5-element phased-array surface coil were used for all CMR studies. Patients were continuously monitored throughout the examination using a single-lead ECG along with pulse oximetry. Patients were positioned supine, head first. Every image was acquired through ECG gating and during suspended respiration. Left ventricular (LV) function was assessed by a standard steady-state free precession technique (repetition time 3.3ms; echo time 1.7ms; flip angle 60°; matrix 192×256; echo-train length 23; slice thickness 8mm; 20 phases). Subsequently, a T2-weighted, triple inversion-recovery fast spin-echo sequence (T2 STIR) (repetition time 2000ms; echo time 100ms; matrix 256×512; slice thickness 8mm; echo-train length 33) covering the whole ventricle was carried out to determine the area at risk. Finally, late enhancement (LE) imaging was performed 5min after the administration of 0.1 mmol/kg of gadobutrol (Gadovist, Bayer Schering Pharma, Berlin, Germany) using a 3-dimensional inversion recovery turbo gradient echo sequence (repetition time 4.1ms; echo time 1.23ms; flip angle 15°; matrix 256×256) and covering the entire myocardium once again. Inversion time was determined to null normal myocardium on an individual basis.
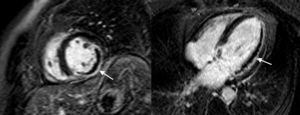
Image AnalysisImage analysis was performed by an expert CMR radiologist on an independent workstation provided by the manufacturer (View-Forum release 6.3, Philips Medical Systems). The endocardial border was determined in end-systole and end-diastole for all short axis images to analyze ventricular volumes–expressed in milliliters. Presence or absence of microvascular obstruction (MVO) was also assessed and defined as lack of contrast on arrival at the core of the infarcted area in LE imaging (Figure 1). The MVO was coded dichotomously (MVO positive vs MVO negative): MVO was positive when it was present in at least 1 segment. A region of interest was drawn within a normal myocardial segment after manually tracing epicardial and endocardial contours in both T2 STIR and LE images. We identified a myocardial region as “affected” when there was more than mean +2 standar deviations (SD) of normal established myocardium in T2-weighted images and +5 SD in LE images. Subendocardial hyperintensity was often found in the most apical segments, and it was excluded by manually tracing the endocontour.
Figure 1. Short axis and 4-chamber view late gadolinium images of a patient with an extensive, transmural infarct in the left ventricle inferior and lateral walls with a concomitant important zone of microvascular obstruction (arrows).
Myocardial necrosis was defined by the extent of abnormal LE; myocardium at risk was defined by extent of edema; and the quantity of salvaged myocardium resulted from the difference between myocardium at risk and myocardial necrosis. Both infarct size and the area at risk were expressed as percentages of the total LV mass. The MVO areas were manually included both as myocardium at risk and infarcted myocardium. Myocardial salvage index (MSI) resulted from the following fraction: (area-at-risk minus infarct size)/area-at-risk.
The intra-observer agreement was 0.96 with a kappa value of 0.88 (95% confidence interval [CI] 0.67 to 1.0) for necrosis area and 0.93 with a kappa valued of 0.84 (95% CI 0.59 to 1) for the area at risk when analyzing the first 20 patients.
Statistical AnalysisContinuous variables were tested for normal distribution applying the Kolmogorov-Smirnov test. The normal distributed continuous variables are shown as mean±SD, and those nonparametrically distributed as a median with an interquartile range. Discrete variables are presented as frequencies (percentages), subsequently compared using Chi-square or Fisher's tests (where appropriate). Student's t-test was applied for continuous variables.
All tests were 2-tailed and P values<.05 were considered statistically significant. We used the SPSS software (version 15.0, SPSS Inc, Chicago, Illinois, United States) to perform the analyses.
RESULTSOnly 50 of the 63 patients consecutively selected were finally included, as 13 patients were excluded for not fulfilling all the criteria: claustrophobia (2), clinical instability or exitus after rescue PCI (3), pacemakers or implantable defibrillator devices (1), previous AMI (5), inability to dilate the guilty vessel (1) and follow-up loss (1). The baseline characteristics of the 50 patients who finally formed the study population are shown in Table 1; 68% of them were Killip-Kimball class 1 on admission.
Table 1. Baseline Characteristics of the Study Population and Procedural Data of the Rescue Percutaneous Coronary Intervention.
| Patients | 50 |
| Mean age (years) | 58.5±11.4 |
| Male | 39 (78%) |
| Cardiovascular risk factors | |
| Diabetes mellitus | 15 (30%) |
| Hypercholesterolemia | 25 (50%) |
| Smoking | 29 (58%) |
| Hypertension | 23 (46%) |
| Location of the STEMI | |
| Anterolateral | 29 (58%) |
| Posteroinferior | 21 (42%) |
| TIMI flow grade before PCI | |
| <3 | 34 (68%) |
| 3 | 16 (32%) |
| TIMI flow grade after PCI | |
| <3 | 4 (8%) |
| 3 | 46 (92%) |
| Culprit vessel | |
| Left anterior descending | 27 (54%) |
| Circumflex artery | 5 (10%) |
| Right coronary artery | 18 (36%) |
| Multivessel disease | 27 (54%) |
| LVEF in angiography (%) | 51.7±12.1 |
| Thrombus aspiration | 18 (36%) |
| Glycoprotein IIb-IIIa inhibitor | 12 (24%) |
| PCI treated with stent | 49 (94%) |
| Number of stents implanted | 1.2±0.7 |
| Use of drug-eluting stents | 20 (40%) |
| No reflow during PCI | 5 (10%) |
| Improved ST segment elevation post-PCI, | 50% |
LVEF, left ventricular ejection fraction; PCI, percutaneous coronary intervention; STEMI, ST elevation myocardial infarction; TIMI, Thrombolysis in Myocardial Infarction.
Data are presented as n (%) unless otherwise noted.
The main angiographic findings are listed in Table 1. Of these patients, 68% presented TIMI 0-2 flow, with coexisting thrombus in 62% of the cases. Eighteen patients (36%) went through percutaneous thrombectomy. Stents were implanted in 47 of them (94%); drug eluting stent were used in 40% of PCI rescue cases. A TIMI 3 flow was achieved in 92% of the patients and an improved ST segment in 50%. No clinical events occurred that suggested acute or subacute thrombosis of the stent during the period elapsed between angiographic study and CMR study.
Delay TimesThe different delay times are summarized in Table 2. The mean time elapsed between pain onset and fibrinolitic agent administration (time pain-needle) was 176±113min, the mean time between lysis and rescue PCI being 209±122min. The mean time elapsed between pain onset and PCI was 390±152min, with a median of 355min and an interquartile range of 275-480min. A CMR was performed in all patients within 6 days of the acute event, with a mean of 4.7±1.3 days.
Table 2. Delay Times.
| Pain to lysis time (min) | 176.4±113.3 (60-475) |
| Lysis to rescue PCI (min) | 208.6±121.6 (80-675) |
| Pain to rescue PCI (min) | 389.6±152.2 (181-820) |
| Days until CMR | 4.7±1.3 (2-6) |
CMR, cardiovascular magnetic resonance; PCI, percutaneous coronary intervention.
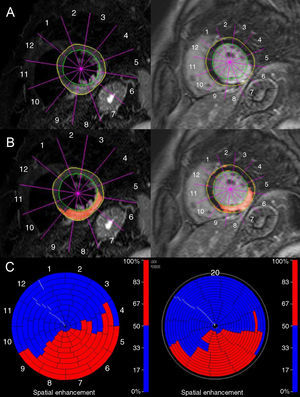
All CMR studies were considered to be diagnostic. The T2-weighted sequence permitted edema detection in all patients after AMI (anterior or lateral 56%, and inferior or posterior 44%). Late gadolinium enhancement images could detect necrosis associated to STEMI in all cases. All patients showed LE in the same edema localization, both facts being compatible with the territory of the guilty coronary vessel. Figure 2 is an example of CMR findings in a patient with an inferior myocardial infarction. Table 3 summarizes the most significant CMR-related findings.
Figure 2. Cardiac magnetic resonance findings in a patient with inferior myocardial infarction. Left: T2-weighted image. There is high signal intensity involving the inferior, inferoseptal, and inferolateral wall of the left ventricle (A and B). In C, color representation of left ventricle edematous myocardial segments (in red). Right: Late enhancement image showing irreversible myocardial injury (A and B) with a very similar extension and distribution to the edematous myocardium. In C, color representation of left ventricle nonviable myocardial segments (in red).
Table 3. Cardiovascular Magnetic Resonance Results.
| Total | TIMI≤2 before PCI | TIMI 3 before PCI | P | |
| Patients, n | 50 | 34 | 16 | |
| Localization | 1 | |||
| Anterior or lateral % (n/N) | 56%(28/50) | 19 | 9 | |
| Inferior or posterior % (n/N) | 44%(22/50) | 15 | 7 | |
| End-systolic volume (ml) | 72.1±32.6 | 77.9±35.8 | 59.2±19.4 | .06 |
| End-diastolic volume (ml) | 146.1±36.3 | 151.1±38.6 | 135.1±28.8 | .16 |
| LVEF (%) | 52.4±10.3 | 50.6±11.1 | 56.4±6.9 | .06 |
| Late microvascular obstruction (% LV) | 39.6% | 48.5% | 20.0% | .11 |
| Area at risk (% LV) | 37.1±13.2 | 40.0±12.9 | 30.7±11.8 | .02 |
| Infarct size (% LV) | 34.3±12.5 | 36.9±12.3 | 28.5±11.3 | .03 |
| Myocardial salvage (% LV) | 3.2±3.6 | 3.3±3.6 | 3.0±3.7 | .80 |
| Myocardial salvage index (%) | 9.0±8.3 | 8.2±6.9 | 10.8±10.9 | .31 |
| Pain to lysis time (min) | 176.4±113.3 | 173.5±100.8 | 182.2±138.3 | .81 |
| Lysis to rescue PCI (min) | 208.6±121.6 | 217.6±145.3 | 190.4±46.9 | .47 |
| Pain to rescue PCI (min) | 389.6±152.2 | 395.2±167.4 | 379.9±121.7 | .73 |
| Days until CMR | 4.7±1.3 | 4.5±1.5 | 5.0±1.0 | .25 |
CMR, cardiac magnetic resonance; LV, left ventricle; LVEF, left ventricular ejection fraction; PCI, percutaneous coronary intervention; TIMI, Thrombolysis in Myocardial Infarction.
Data are expressed as mean ± standard deviation unless otherwise indicated.
The mean edematous area of the total myocardial mass in T2-weighted images was 37±13% (area at risk), the mean enhanced area in LE images being 34.5±13% (infarct size). This yields an absolute difference of 3±4% (salvaged myocardium). In relative terms, only 9±8% (MSI) of the total myocardium at risk (edematous area) was actually salvaged.
In 40% of the cases, MVO was detected, usually located in the subendocardium and showing a variable degree of transmurality. When MVO was present, 58% of the cases had 1 to 3 affected segments, 26% between 4 and 6, and 16%, 7 or 8 segments.
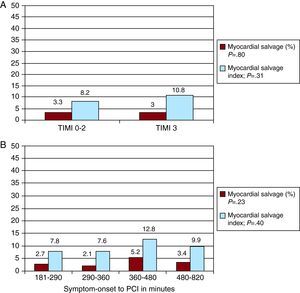
Relationship Between Pre-Rescue Percutaneous Coronary Intervention Thrombolysis in Myocardial Infarction Flow and Myocardial SalvageWe analyzed the extent to which there was a significant difference depending on whether patients had arrived at the catheterization lab with the artery open and a TIMI 3 flow or with the artery occluded and a slow flow (TIMI flow ≤2) (Table 3). Both the area at risk and the final infarct size were significantly higher in the group presenting an occluded artery and/or a slow flow (TIMI 0-2) than in the group with a normal flow (TIMI 3) (40.0±12.9 vs 30.7±11.8, P=.02, and 36.9±12.3 vs 28.5±11.3, P=.03, respectively). Nevertheless, the salvaged myocardium and the MSI were quite similar in both groups (3.3±3.6 and 8.2±6.9 in TIMI 0-2 vs 3.0±3.7 and 10.8±10.9 in TIMI 3; P=.80 and 0.31, respectively). Figure 3A provides all this information.
Figure 3. A, Amount of myocardial salvage and myocardial salvage index according to Thrombolysis in Myocardial Infarction flow before percutaneous coronary intervention. B, Amount of myocardial salvage and myocardial salvage index according to time from symptom onset to reperfusion (quartiles).PCI, percutaneous coronary intervention; TIMI, Thrombolysis in Myocardial Infarction.
Link Between Delay Time and Myocardial SalvageThe delay time was divided in quartiles for the purpose of analyzing the influence on the quantity of salvaged myocardium and MSI. No significant difference was observed between those patients who went through rescue PCI within a shorter time and those with longer delay times. Figure 3B shows the amount of myocardial salvage and MSI according to the time elapsed between the first symptoms and reperfusion. There were no relevant statistical differences between these 4 groups.
No relationship was found between the ST resolution and the quantity of salvaged myocardium (P=.36) or MSI (P=.24). Nor was any association found between the presence of TIMI 3 flow after PCI and the quantity of salvaged myocardium (P=.45) or MSI (P=.43).
DISCUSSIONTo our knowledge, this is the first study where the salvaged myocardium with CMR after performing rescue angioplasty has been analyzed. We are in a position to state that the benefits in terms of salvaged myocardium are very low when fibrinolysis fails and the patient is directed towards a rescue angioplasty strategy. The main reason for the almost nonexistent benefit lies in the long period elapsed until the artery is opened with an effective flow.
Rescue PCI is usually employed to restore a TIMI 3 flow when fibrinolytic treatment has not been successful. This strategy has always failed to provide benefits in terms of mortality reduction, the recommendation level according to current guidelines being IIa.7, 8 However, the amount of myocardium that we were actually “rescuing” had never been quantified. CMR is the only noninvasive technique which can detect the area of infarcted myocardium–both the necrotic area with an irreversible injury and that of the associated edema. This study offers the possibility of evaluating these indexes in a new scenario such as rescue PCI, which in turn permits us to examine the difference between both areas and, ultimately, the “salvaged myocardium.”
The edema in these patients is a transitory finding, almost constant in early phases after an AMI, which had been already demonstrated in studies performed both in animals12 and in human patients13 with the extension of transverse relaxation time (or T2) resulting in an increased signal contrast between the edematous and normal myocardium in T2-weighted sequences.14 The mean myocardial salvage in our series is 3±4%, very low if we compare it with the 14% to 18% of myocardial salvage in primary PCI9, 15, 16 for similar-sized infarcts (the area at risk in our series was 37% vs 35.2% in Eitel's study16). The same can be said about MSI: 8.8±8 in our patients with rescue PCI compared to 48.3 provided by studies with CMR in primary PCI.16
What has been said above could explain the limited clinical benefit that rescue PCI has provided at different clinical trials5, 6–and even in a meta-analysis17–with no resulting reduction in the mortality rate. Thus, for example, the Wijeysundera meta-analysis17 did not reveal any mortality rate reduction with rescue PCI compared to medical therapy (10.4% to 7.3%, relative risk [RR] 0.69 [95% CI 0.36 to 1.05]; P=.09), though lower re-infarction rates were actually observed from 10.7% to 6.1% (RR 0.58 [95% CI 0.35 to 0.97]; P=.04), which is clearly linked to target vessel opening.
This scarce salvaged myocardium clearly has to do with the time which passes before the artery is opened. That time is quite homogeneous and always high in our study group (the mean is 390±152min and the interquartile range 275-480min), which means that there are no patients with a medium or high benefit. Logically, this long delay correlates with a series of time periods intrinsic to rescue PCI: time from symptom appearance to first medical contact; time from first medical contact to the start of fibrinolysis; the necessary 90min after starting the fibrinolytic therapy to observe resolution <50% of ST-segment; the time needed to transfer the patient to the catheterization lab and the time elapsed between arrival at the lab and balloon inflation. Primary angioplasty has actually achieved a reduction in those times with different programs and action strategies worldwide,18 always based on the creation of on-call interventional cardiologist teams coordinated through dynamic and agile transportation systems. Unfortunately, unlike what happens in primary PCI,18 delay times in rescue PCI can only be slightly improved, as is easily confirmed when we see that delay times in our surroundings (real life) are very similar to those found at clinical trials, where the time elapsed between symptom appearance and the beginning of the treatment ranged from 258 to 414min.17
The pharmaco-invasive strategy (early thrombolytic infusion followed by routine nonimmediate angioplasty) has recently supplied some very promising data in terms of infarct size and MVO.19 In fact, it could be a suitable option for patients without serious complications, especially in areas located far away from tertiary hospitals.
It is worth highlighting that this scarce benefit with rescue PCI is uniform. Firstly, patients arriving earlier (quartiles 1 and 2) do not obtain any more benefits than those who arrive later. The fact that the time from symptom appearance to reperfusion in the first quartile ranged from 181 to 290min explains the limited myocardial salvage (2.7%) in the best of cases. And these times are far from those marked by some authors as hypothetical to determine a clear benefit in terms of mortality reduction and myocardium salvage.20 Secondly, the fact of arriving with the artery open (TIMI 3) does not seem to have a more positive effect (MSI 10.8 vs 8.2 in TIMI 0-2; P=.31). This could happen because artery opening occurs too late (too much time elapses between the request of rescue PCI and the moment in which angioplasty is performed) and also by the possible injury caused by reperfusion due to embolization in arteries with a high thrombotic component; this embolization could actually explain to some extent why only 50% of patients presented a clear ST segment improvement.
The failure of fibrinolysis in a high percentage of patients (30%-50%) who will undergo rescue angioplasty with such little benefit should be another pillar for the development and consolidation of primary angioplasty as the elective technique for reperfusion in STEMI patients. Therefore, an effort should be made to actively promote programs such as “Stent 4 Life”21–sponsored by the European Society of Cardiology–which has as its primary objective “to implement an action program meant to increase patient access to primary PCI where indicated.” This will surely help to prevent a scenario where almost half of the reperfused patients with fibrinolysis can hardly obtain any benefits in terms of salvaged myocardium.
LimitationsAlthough we completed the protocol in this prospective study, it took place in a single center and the number of patients was small (n=50). Therefore clinical conclusions cannot be reached, which obviously was not the objective of the study. The delay in performing CMR could favour edema resorption and thus reduce the difference between area at risk and infarct size. Nevertheless, the time frame for CMR performance in our study was 6 days, which leads us to assume that this situation should not yet have taken place. Another limitation that needs to be mentioned is the exclusion of hemodynamically unstable patients who could not tolerate CMR in the days following AMI. In fact, we had to add the patients deceased days after rescue PCI. This explains why 68% of our patients were STEMI Killip-Kimball 1. Thus, the salvaged myocardium in the AMI with worse hemodynamic tolerance and higher mortality both in the short and in the long term were not really examined, although the amount of salvaged myocardium in those patients was most probably even lower than among the patients actually examined in this study. As a final consideration, it is necessary to underline that we do not know the percentage of patients who underwent fibrinolysis and were later referred for rescue PCI. We only have precise information available for 3 of the 7 hospitals which referred 32% of the patients after fibrinolysis.
CONCLUSIONSThe myocardial salvage after rescue PCI quantified by CMR is very small. The long time elapsed between pain onset and the opening of the infarct-related artery with PCI (6.5h on average) is most probably the reason for the minimal effect produced by rescue PCI. Such long delay times–which are additionally very hard to improve–make the low benefit of rescue PCI visible with CMR uniform in this whole group of patients. Primary PCI programs should be promoted in order to improve the benefits obtained by a high percentage of patients referred for the pharmacological reperfusion strategy.
CONFLICTS OF INTERESTNone declared.
Acknowledgements
We gratefully acknowledge all the cardiologists and intensive care physicians from Alicante province for their cooperation in the performance of this study.
Received 22 January 2011
Accepted 28 April 2011
Corresponding author: Departamento de Cardiología, Hospital General Universitario de Alicante, Pintor Baeza 12, 03010 Alicante, Spain. ruiz_jmi@gva.es