Keywords
INTRODUCTION
In recent years, the key role of the inflammatory process, and activation of the cellular immune system in particular, has been recognized in the pathogenesis of coronary atherosclerosis and in triggering acute coronary syndrome (ACS). Several findings have demonstrated that inflammatory cells of the monocyte-macrophage system participate from the earliest stages in the genesis of atherosclerotic lesions, and their activation significantly contributes to progression and destabilization.1 Furthermore, in ACS, there is increased monocyte/macrophage activation which is associated with an increased risk of recurrence.2,3
Neopterin, a metabolite derived from the guanosine triphosphate-biopterin synthetic pathway, is an excellent biomarker of cellular immune system activation.4 Monocytes and macrophages, upon stimulation by gamma interferon (IFN) secreted by activated type I helper T cells, are the most important source of neopterin in humans.5 When the cellular immune system is activated, as occurs in numerous diseases with an autoimmune and infectious etiology, as well as in graft rejection, considerably high concentrations of neopterin may be found in serum and other organic fluids. Thus, its determination is a useful tool for following up disease activity and any response to treatment.6 Similarly, high neopterin concentrations have been demonstrated in patients with acute and chronic coronary syndrome.7 This finding is not only associated with the extent and severity of coronary atherosclerotic disease,8,9 but also correlates with the degree of atherosclerotic plaque vulnerability and with acute inflammatory activity in atherosclerosis.10,11b Thus, neopterin is currently considered to be good biomarker of coronary arterial disease.12
It is a well-known fact that the occurrence of ACS events is not distributed evenly throughout the day, but undergoes cyclic variations. Thus, for example, it has been clearly demonstrated that the onset of acute myocardial infarction (AMI) most frequently occurs in the early hours of the morning.13 Studies conducted on the bioperiodicity of the immune response in healthy subjects show that both the number and functions of immune system cells, as well as the degree of activity of inflammatory processes, can vary over the course of the day.14 However, there are few data on the likelihood that the cellular immune system can fluctuate cyclically in patients with ACS. The aim of this study was to investigate how the serum concentration of neopterin varies during the light and dark phases of the day in patients with ACS with persistent ST-segment elevation acute coronary syndrome (STEACS) who had undergone primary angioplasty. Concentrations were compared in patients with STEACS to those obtained in a control group without clinical evidence of atherosclerotic disease in order to investigate whether the light-darkness response patterns were different. The basic hypothesis of our study was that patients with STEACS who had undergone primary angioplasty would present significantly higher serum neopterin concentrations than control subjects and that these concentrations also vary during the course of the day in patients with STEACS.
METHODS
Study Subjects
From January 2001 to December 2002, 155 patients diagnosed with AMI were cared for in the Coronary Care Unit (CCU) of the Hospital Universitario de Canarias, Spain. From this population, patients were selected who presented STEACS who had undergone primary angioplasty as reperfusion therapy. Similarly, during the same period, a control group was selected made up of subjects of similar age and sex, without a history of disease, who were not taking medication or addicted to drugs and without evidence of heart, vascular, metabolic, neoplastic, or inflammatory disease determined through the careful study of case histories and physical examination, chest x-ray, basal electrocardiogram, and standard blood and urine analysis. All study subjects were screened for diabetes mellitus, and only non-diabetic subjects were accepted under the inclusion criteria. Exclusion criteria included a course or history of peripheral arterial disease, specific and nonspecific infectious disease, autoimmune and connective tissue disease, malignant diseases, addiction to drugs, immunosuppressive treatment, radiation therapy or chemotherapy, acute or chronic kidney failure, and liver disease. All subjects gave informed consent before the beginning of the study. The study was approved by the ethics committee of our institution.
Study Protocol
The study subjects, both patients and controls, were studied in the CCU in strictly controlled environmental conditions of light and darkness. Each of the CCU rooms in our institution is independent, with a window to the outside, with excellent acoustic and light isolation capability. The environmental light conditions of the CCU during the study were similar to those of the normal external light-darkness cycle. The light phase in the CCU was 14 h (light intensity, 1745 [33] lux) and the darkness phase was 10 h (light intensity, 1.33 [0.3] lux). Light intensity was measured at head height. The lights were switched on and off at 7:00 in the morning and 9:00 at night, respectively. At CCU admission, the patients were fitted with a heparin lock catheter in the antecubital vein of the forearm for blood extraction. A sample was extracted for standard analytical assessment according to the diagnostic protocols of our CCU. All the subjects rested throughout the study. To determine the serum concentrations of neopterin, blood samples were extracted at 10:00 in the morning (light phase) and 3:00 at night (darkness phase). In patients with ACS, these samples were collected in the first 24 h of the onset of symptoms. The blood sample taken at 3:00 at night was extracted by a specially trained nurse, in darkness, with the aid of a small flashlight emitting weak red light (light intensity, <30 lux), at all times avoiding directing the beam toward the face of the subjects when sleeping. The serum samples, obtained by centrifugation of the blood, were decanted into several aliquot tubes and stored at -80oC until analysis.
Laboratory Methods
The standardized procedures employed by the central laboratory of our institution were used to determine plasma concentrations of total cholesterol, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), triglycerides, and glucose. Troponin I values were determined using the immunoenzymatic method based on the ELISA sandwich technique (Boehringer Mannheim, Germany). Serum concentrations of neopterin were determined using an ELISA method, following the manufacturer's instructions provided in the kits (DRG Instruments, Marburg, Germany). In this ELISA test, the lowest detection limit for neopterin was 0.7 nmol/L and the intra-assay and inter-assay coefficients of variability were 5.3% and 9%, respectively.
Statistical Analysis
Data were analyzed using the SPSS statistical software package 10.0.1 for PC (SPSS, Analytical Software, Chicago, Illinois, USA) and Statistica version 5.0 (StatSoft, Tulsa, Oklahoma, USA). Discrete variables are expressed as percentages and continuous variables are expressed as mean (standard deviation). Verification of the normal distribution of continuous variables was performed using the Kolmogorov-Smirnov test. The Student t test was used to compare continuous variables in patients and controls. The c2 test was used for discrete variables. Variations in neopterin concentrations during light and darkness phases between patients with STEACS and controls were analyzed by MANOVA, using the variable group (patients with STEACS vs controls) as an independent variable and phase (light vs darkness) as a repeated-measures dependent variable. A P value less than .05 was considered significant.
RESULTS
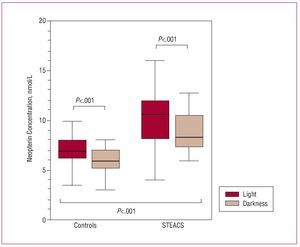
The study sample consisted of 96 patients with STEACS who had undergone primary angioplasty and a control group of 84 subjects with no clinical evidence of heart, vascular, metabolic, neoplastic, or inflammatory disease. In the STEACS patient group, the mean left ventricular ejection fraction was 47% (12%); 11 (11.45%) patients presented multivessel coronary artery disease; and 15 (15.6%) presented symptoms of left ventricular failure, characterized by Killip class 1. The remaining clinical characteristics and baseline analytical parameters of the study subjects (patients with STEACS and controls) are shown in Table 1 and Table 2. As shown in Table 1, there were no significant differences between patients and controls in age, sex, and the distribution of risk factors for coronary artery disease. Table 2 shows the concentrations of total cholesterol and its different fractions, triglycerides, glucose, and troponin I. No significant differences were found between patients and controls, except in the case of troponin I that, as expected, presented significantly higher concentrations in the group of patients with STEACS. Figure shows the concentrations of neopterin in patients and controls during the light and darkness phases. In both groups, there were light-darkness variations in serum concentrations of neopterin: mean serum concentrations of neopterin were significantly higher during the light phase than during the darkness phase, both in the control group (light vs darkness, 6.98 [1.96] vs 5.87 [2] nmol/L; P<.001) as well as in the STEACS patient group (light vs darkness, 10.2 [3.8] vs 8.84 [2.22] nmol/L; P<.001).
Figure. Serum neopterin concentrations in light and darkness phases in the group of patients with ST-segment elevation acute coronary syndrome (STEACS) and control group. MANOVA: controls versus STEACS group, P<.001; control group in light phase versus darkness phase, P<.001; STEACS GROUP in light phase versus darkness phase, P<.001.
On the other hand, the patients with STEACS presented significantly higher concentrations of neopterin than controls during the same phases (P<.001). However, no interaction effect was observed between the 2 factors studied: group (patients with STEACS vs control) and phase (light vs darkness) (P=.58).
DISCUSSION
The available information on diurnal variations in cellular immune system activation in patients with atherosclerotic coronary arterial disease is scarce. The main finding of this study is that of light-darkness variations in the neopterin production in patients with STEACS who had undergone primary angioplasty.
The cellular immune system plays a key role in the inflammatory response that leads to the rupture of atherosclerotic plaque and the triggering of ACS.15 There is an increase in the number and activity of T cells in unstable atherosclerotic plaques and of type 1 helper T cells that produce IFNg in the peripheral blood of patients with atherosclerotic coronary arterial disease, and in particular in those with ACS.16,17 Thus, serum concentrations of neopterin would indicate, in each individual, the degree of activation of the cellular immune system. In line with this, previous studies have demonstrated that patients with ACS present significantly higher neopterin concentrations in peripheral blood than normal subjects.7-9 Our results agree with theirs and show that neopterin concentrations are significantly higher in the patients with STEACS than in controls in both light and darkness phases. In patients with ACS, the degree of the increase in neopterin concentrations correlates with the presence of complex and vulnerable lesions and their number.8,10,11 Furthermore, in patients with stable coronary arterial disease, neopterin concentrations are higher in those who contract a more rapidly progressing disease.18,19 On the other hand, it has been demonstrated that serum neopterin concentrations are not correlated with serum concentrations of biomarkers of myocardial necrosis.20 Thus, in patients with STEACS, the increase in neopterin, rather than representing an immune response to myocardial injury, indicates the degree of cellular immune activity in the disease and the vulnerability of atherosclerotic lesions. We have to point out that, in the study subjects, the distribution of the proatherogenic risk factors was similar between the groups. This makes it unlikely that a difference in the prevalence of these factors could explain the differences found in neopterin concentrations between the two groups and in the phases studied.
Circadian variations at the onset of clinical manifestations of cardiovascular disease may indicate that they could be instigated, in some way, by physiological rhythms or events that present similar temporal organization, with "peaks of activity" at a given time of the day or night.21,22 Thus, diurnal variations that could help to trigger thrombotic events have been demonstrated in blood pressure, heart rate, humoral factors such as increased catecholamines, platelet aggregation, and the reduction of tissue plasminogen activator in the early hours of the morning.23 Furthermore, Tanaka et al24 have recently shown that there are circadian variations in the incidence of atherosclerotic plaque rupture.
In humans, inflammatory processes and the immune response present circadian rhythms. These events are controlled by the central neuroendocrine system,25 specifically the pineal gland, by differential secretion under light and darkness conditions of its main hormonal product, melatonin.26 Born et al27 have demonstrated circadian fluctuations in the number of the different types of circulating immune cells and that each cellular subtype has a peak in peripheral blood that varies according to the time of day. In particular, the number of monocytes and various T lymphocyte subtypes reach their peak value during the dream period of the darkness phase. On the other hand, it has been shown that the production of proinflammatory cytokines has a diurnal rhythm.28 Our group has demonstrated light-darkness variations in proinflammatory cytokine production in patients with AMI,29 in addition to diurnal variations in serum concentrations of some inflammation markers, such as interleukin-6,30 C-reactive protein,31 and other mediators, such as matrix metalloproiteinase-9.32
Diurnal variations in neopterin production has been previously described in healthy subjects.33,34 However, the results of our study extend this finding to patients with ACS. Auzéby et al33 have demonstrated circadian rhythm in urinary neopterin in healthy subjects. The authors report that the excretion of urinary neopterin peaks during the early morning, and point out that this would indicate the previous activation of circulating T lymphocytes.35 In contrast to these authors, our group suggests that diurnal variations in serum neopterin concentrations in healthy subjects could be influenced, at least partly, by the melatonin circadian rhythm.34
In our opinion, the finding of diurnal variations in cellular immune system activation in patients with ACS may be important for several reasons. First, it contributes relevant information that aids in understanding the pathophysiological mechanism that could explain, at least partly, diurnal variations when patients present with ACS. Second, diurnal variations in neopterin production and of other inflammation markers should be taken into account not only when planning when to extract blood for diagnostic purposes, but also when designing future studies on inflammation in patients with ACS.36 Finally, the fact that immunologic functions in ACS have a diurnal rhythm suggests that the immune response could be modified and therapeutically manipulated. This could have implications when optimizing treatment.
Nevertheless, our study has some limitations. This was a case-control study with a relatively small sample. Due to not having performed serial determinations during the day and night, it cannot be affirmed that there is a circadian rhythm in neopterin production. However, the differences found in serum neopterin concentrations in the light and darkness phases, although of limited magnitude, are sufficiently significant to be able to affirm diurnal variations in their production and, thus, in the degree of cellular immune system activation in patients with ACS. An important limitation that should be taken into account in relation to this study is that endogenous neopterin production can be increased in stressful situations. Although this factor could largely explain the observed increase in the first 24 h of AMI, it has to be pointed out that the observed increase in urinary neopterin concentrations under emotionally stressful conditions has been described as occurring from 36 to 84 h later.37,38 On the other hand, we determined neopterin concentrations in peripheral venous blood samples, rather than in blood from the coronary sinus. Thus, we have assumed that serum neopterin concentrations reflect the inflammatory process that occurs in area of the coronary artery. In this regard, a previous study by Fyfe et al39 demonstrated that there were no significant differences in neopterin concentrations when this was determined in blood from the coronary sinus versus those found in blood from the superior vena cava.
CONCLUSIONS
The results of our study demonstrate diurnal variations in serum neopterin concentrations in patients with STEACS who had undergone primary angioplasty and thus it can be stated that there are diurnal variations in the activation of the cellular immune response in these patients. The importance of this finding resides in its contribution to understanding the pathophysiological mechanism that could explain, at least partly, diurnal variations when a patient presents with ACS. Nevertheless, further studies are needed to help clarify the mechanisms that underlie the periodicity observed in the presentation of the clinical manifestations of cardiovascular disease. This may contribute to designing intervention strategies that could provide better protection at the time of greatest risk.
ACKNOWLEDGEMENTS
The authors wish to thank Alejandro Jiménez Sosa of the research unit of the Hospital Universitario de Canarias, Spain, for his help with advice on statistics during manuscript revision.
ABBREVIATIONS
ACS: acute coronary syndrome
AMI: acute myocardial infarction
CCU: coronary care unit
IFNg: interferon-gamma
STEACS: ST-segment elevation acute myocardial infarction
Correspondence: Dr. M.J. García-González.
Unidad de Coronarias. Servicio de Cardiología. Hospital Universitario de Canarias.
Ctra. La Cuesta-Taco. Ofra, s/n. San Cristóbal de La Laguna.
38320 Santa Cruz de Tenerife. España.
E-mail: mjgg181262@hotmail.com
Received December 15, 2007.
Accepted for publication August 5, 2008.