Keywords
INTRODUCTION
Right-sided endocarditis is a well-defined clinical entity in patients with a history of intravenous drug use (IVDU) or who have a pacemaker or other intracardiac device.1-4 Its clinical presentation and treatment differ from those of left-sided endocarditis,1-4 and its prognosis is more favorable, as many patients can be cured with medical treatment alone1,4 or with surgical device extraction in pacemaker endocarditis.2-3 Fever associated with a history of IVDU or use of a pacemaker should raise suspicions of endocarditis, which can be confirmed with blood cultures and echocardiography.
Isolated right-sided endocarditis in the absence of a history of IVDU or in patients without a pacemaker is a poorly-understood entity. Most published reports of this disease consist of old descriptions of single cases.5-7 The largest series, described by Nandakumar et al,8 summarized the characteristics of 29 cases of tricuspid valve endocarditis in non-IVDU patients published by various authors during the last decades of the previous century. The study by Naidoo9 analyzed 15 cases of right-sided endocarditis in non-IVDU patients; however, this was a retrospective analysis that also included cases of left-sided endocarditis.
Information is thus lacking on the clinical profile and prognosis for right-sided endocarditis in patients without a pacemaker and without a history of IVDU. The aim of the present study was to investigate the current frequency of this entity and describe its clinical, microbiological, echocardiographic, and prognostic profile.
METHODS
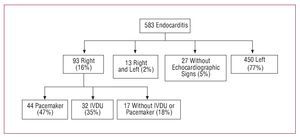
We analyzed 583 episodes of endocarditis consecutively diagnosed between 1996 and 2006 in the cardiology and cardiac surgery unit at four tertiary centers in accordance with the Duke criteria (to 2002) or modified Duke criteria10 (2003 to 2006). Of the 93 episodes located in the right side of the heart (16%), 44 occurred in an intracardiac device (47%), 32 occurred in patients with IVDU (35%), and 17 in patients with no history of IVDU and who did not have a pacemaker (18%). These 17 patients constituted the study group for the present report (2.9% of the total cohort in our series). Figure 1 shows the distribution of our population.
Figure 1. Distribution of our population. IVDU indicates intravenous drug use.
For each patient we used the same protocol to record information prospectively starting on the day of hospital admission. The protocol covered 90 variables11: 19 epidemiologic, 8 clinical, 10 analytical, 4 radiographic, 6 electrocardiographic, 13 microbiological, 16 echocardiographic, and 14 follow-up characteristics. For all patients we recorded the findings from at least 1 physical examination, 1 electrocardiogram, 1 chest x-ray, 1 urinalysis, 3 blood cultures on admission, 3 blood cultures 48 hours after the start of antibiotic treatment, and 1 transthoracic and transesophageal echocardiogram. Empirical antibiotic treatment was started after blood cultures were taken if the patient's clinical status so required, and treatment was amended if necessary according to the results of blood cultures and the antibiogram. If blood cultures after 72 hours were negative, specific serological tests were done for Chlamydia, Brucella, Q fever, Legionella, and Mycoplasma.
Definition of Terms
Health care-associated infective endocarditis was defined as either nosocomial infection or nonnosocomial health care-associated infection. Endocarditis was considered nosocomial when developed in a patient hospitalized for more than 72 hours prior to the onset of signs and symptoms consistent with endocarditis. Nonnosocomial health care-associated infective endocarditis was defined as endocarditis in outpatients with extensive health care contact (permanent devices for intravenous treatment, hemodialysis catheters, etc). Episodes diagnosed at the time of admission (or within 72 hours of admission) in patients not fulfilling the criteria for health care-associated infection were considered community-acquired.
We considered early prosthetic endocarditis as that occurring within the first year after surgery.12 The disease was considered acute onset when the time between appearance of symptoms and admission was no longer than 15 days. Renal failure was defined as the presence of serum creatinine concentration higher than 2 mg/dL. Chronic anemia was defined as a hemoglobin concentration <9 g/dL documented during at least 1 year before hospitalization. We considered any treatment able to diminish cellular or humoral immunity to constitute immunosuppressive treatment. Echocardiographic criteria for vegetations, abscess, pseudoaneurysm, and fistula were as described in an earlier publication.13
Indications for surgery included heart failure refractory to medical treatment, fungal endocarditis, repeated embolism with persistent vegetations on echocardiography, and uncontrolled infection, defined as persistent bacteriemia or fever of more than 7 days' duration despite appropriate antibiotic treatment, once other foci of infection were ruled out.
Statistical Analysis
We report a descriptive analysis of epidemiologic, laboratory, radiographic, electrocardiographic, microbiological, echocardiographic, and follow-up variables in 17 patients without IVDU and without a pacemaker who had isolated right-sided endocarditis. Continuous variables are expressed here as the mean (standard deviation) or median and interquartile range, and categorical variables are reported as the absolute value and percentage. All data were analyzed with SPSS software, v. 12.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
Epidemiological and Clinical Findings
Mean age was 38 (15) years (range, 20 to 61 years), and 11 patients were men. Infection was nosocomial in 7 patients, community-adquired in 7 and nonnosocomial health-care associated in 3. The port of entry was determined for 11 patients as: intravascular catheter in 6 patients, genitourinary procedures (curettage for septic abortion) in 3, previous cardiac surgery (aortic valve replacement) in 1, and septic shoulder joint effusion in 1. Antecedents of heart disease were documented in 4 patients: 2 had a prosthesis (1 aortic mechanical prosthesis and 1 biological tricuspid valve prosthesis), and 2 had congenital heart disease (ventricular septal defect in 1, and tetralogy of Fallot in 1). Three patients had a history of previous episode of endocarditis (1 aortic endocarditis and 2 tricuspid endocarditis). Predisposing diseases or disorders were found in 8 patients (47%): immunodepression in 4, chronic anemia in 3, diabetes mellitus in 3, immunodepressive treatment in 3, chronic renal failure in 3, and cancer in 3. In 5 patients there was more than one predisposing disease. In most patients the onset was acute, and only 2 patients had symptoms of more than 2 months'duration. In 13 episodes (76%) the patient had received antibiotic treatment prior to the diagnosis of endocarditis, to treat febrile syndrome usually attributed to respiratory infection.
The most frequent symptoms on admission were fever (n=15) and dyspnea (n=5). Three patients were admitted in septic shock, and 3 others had clinical signs of right heart failure. Chest x-ray showed cardiomegaly in 9 patients, septic pulmonary embolism in 7, pleural effusion in 6, and left heart failure in 2.
Microbiological Findings
Blood cultures were positive on admission in 14 patients, and became positive within 48 hours in 1 patient. In 2 patients no causal microorganism could be identified, both were receiving antibiotic treatment when blood cultures were obtained. After 48 hours of antibiotic treatment, blood cultures became negative in 13 patients. The microorganism isolated most frequently was Staphylococcus aureus (n=7; 41%), (methicillin-sensitive in 4 patients, methicillin-resistant in 3). Table shows the microbiological profile of our 17 patients.
Echocardiographic Findings
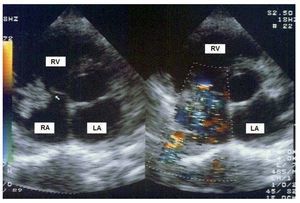
Endocarditis was located on the tricuspid valve in 14 patients (82%). In the remaining patients it was located in the superior vena cava, in a ventricular septal defect, and as early prosthetic endocarditis on a biological tricuspid valve prosthesis. None of the episodes involved more than one valve. All patients had vegetations on transesophageal echocardiogram, and most vegetations were large—(13 [7]) mm ´ (8 [6]) mm; area, 87 (65) mm2—and highly mobile (Figure 2). However, the appearance of perivalvular complications was rare (3 fistulas). Valve rupture occurred in 1 case, and severe tricuspid valve regurgitation in 12 patients.
Figure 2. Transthoracic echocardiogram in a patient with tricuspid valve endocarditis. Large vegetation (arrow) that cause severe valvular regurgitation. LA indicates left atrium; RA, right atrium; RV. right ventricle.
Clinical Course
During hospital stay, left heart failure developed in 1 patient (because of fluid overload in a patient on hemodialysis with diminished left ventricular function), 2 patients developed de novo renal failure, and 1 patient developed complete atrioventricular block.
Anemia was frequent in our patients, and only 3 had hemoglobin concentration on admission higher than 12 g/dL. In 10 patients the concentration was <10 g/dL, and in 7 the value was <9 g/dL. Microscopic hematuria was also common, occurring in 8 patients (44%).
Heart surgery was needed in 5 patients (29%): 3 for recurring pulmonary embolisms despite appropriate treatment, with persistence of large, mobile vegetations on echocardiography, and 2 for persistent infection (1 patient also had right heart failure that failed to respond to treatment). Surgery consisted of debridement and tricuspid valve repair in 3 cases, and valve replacement in 2 cases (1 mechanical prosthesis and 1 biological prosthesis). One patient required implantation of a permanent epicardial pacemaker because of complete atrioventricular block.
Median duration of the hospital stay was 37 days (IQR, 30-63 days). During hospitalization, 2 patients died, both from septic shock. One patient died 24 hours after diagnosis after a 1-month history of febrile syndrome that was treated unsuccessfully outside the hospital. The other patient had metastatic cancer and terminal kidney failure, and reported febrile syndrome of 2 months' duration treated unsuccessfully with antibiotics prior to diagnosis. In-hospital mortality was thus 12%. With regard to the prognosis, mortality in non-IVDU patients without a pacemaker who had isolated right-sided endocarditis was lower than in the group with left-sided endocarditis (11% vs 33%; P=.06)
Post-discharge follow-up of 11 patients lasted a median of 344 days (range, 180-1227 days). One patient died 1 month after discharge from unknown causes, and 1 patient with tricuspid valve endocarditis due to Propionibacterium acnes relapsed after 3 months; all other patients remained free of symptoms. Table summarizes the clinical characteristics in our 17 patients.
DISCUSSION
Right-sided endocarditis is often associated with intracardiac devices or a history of IVDU, and many published studies have reported these 2 forms of presentation of the disease.1-4 However, the appearance of right-sided endocarditis in a patient with neither of these 2 antecedents has rarely been reported, probably because of the low incidence of the disease in such patients. In fact, our search of the literature located only older reports of single cases.5-9 The present study reports findings in the first prospective series to date of non-IVDU patients without a pacemaker who had right-sided endocarditis.
Although the frequency of right-sided endocarditis in the type of patient reported here was low considering the entire group of patients with endocarditis (18% of all patients with right-sided endocarditis, 3% of all patients with endocarditis in our series), the frequency was higher than initially expected, especially in light of the fact that more than half the cases occurred in apparently healthy patients with no predisposing disease. The actual frequency is probably higher than we found, for 2 reasons. The manifesting symptoms of right-sided endocarditis are similar to those of respiratory infection (fever, dyspnea, and pulmonary infiltration), making misdiagnosis likely. Also, because the clinical condition improves with antibiotic treatment, many cases of right-sided endocarditis remain undiagnosed. This explains why most patients in our series received antibiotic treatment before they were correctly diagnosed. Moreover, the actual incidence of the disease may be underestimated because most studies have involved patients with complex endocarditis or whose disease showed a poor clinical course, and who were therefore referred to reference centers.1,8 This issue might represent a selection bias, so that our conclusions should only be pertinent to populations similar to ours.
In general, isolated right-sided endocarditis in non-IVDU patients without a pacemaker occurs in young patients.9,14 The presence of one or more predisposing factors (immunodepression, renal failure or cancer) is not infrequent. In such patients, fever does not usually suggest a diagnosis of endocarditis, but instead points toward other types of infection (eg, respiratory or abdominal), and this can further delay the diagnosis. Right-sided endocarditis should therefore be included in the differential diagnosis in young patients with fever and a predisposing disease.
In most cases the port of entry of the infection could be identified. The most frequent port was via an intravascular catheter, and in fact the possibility of developing endocarditis because of a central venous catheter was noted previously.15 Vascular catheters are the main source of bacteriemias, especially infections caused by staphylococci,16 which were the microorganisms found most frequently in our series.
Delays in the diagnosis are the result, in part, of the fact that the Duke criteria are not appropriate for patients with right-sided endocarditis (ie, the sensitivity of these criteria for detecting the disease is low), and according to many authors the criteria need to be modified.17,18 A series of major and minor criteria have been proposed for the diagnosis of right-sided endocarditis.17 Right-sided endocarditis is not manifested clinically with the classic signs of left endocarditis,5 but with respiratory symptoms. Chest x-ray will frequently reveal septic embolisms and pleural effusion. Because respiratory signs and symptoms predominate, patients are often diagnosed as having pneumonia.5,8 The combination of pulmonary infiltrates and renal disease may lead to a suspicion of vasculitis.8 Pulmonary embolisms are frequent in patients with right-sided endocarditis and might delay the diagnosis of the disease if they are considered as cause of the endocarditis instead of its manifestation. Fever is also a common finding within the first days after a pulmonary embolism, but is rarely very high and prolonged. Therefore, in patients with pulmonary embolism and high, mantained or recurrent fever or with high levels of inflammatory markers, other etiologies of the febrile syndrome should be ruled out, for example right-sided endocarditis, specially in patients with risk factors (central venous catheters, alcoholism, cancer, previous heart disease, etc).19
It has been suggested that right-sided endocarditis should be suspected in the presence of the so-called "tricuspid syndrome": recurrent respiratory events, anemia, and microscopic hematuria.8 The present study corroborates this association, as 24% of our patients had the tricuspid syndrome, and 65% had at least 2 of its 3 components. The appearance of renal alterations in patients with infective endocarditis is frequent, and these alterations can manifest as a variety of complications of varying clinical significance. The most common renal manifestation is hematuria or proteinuria (or both), and the most frequent pathological finding is glomerulonephritis, which is usually proliferative and diffuse or vasculitic.20,21
Blood cultures were positive in most patients. In our series the most frequent causal microorganism was Staphylococcus aureus, as also reported in earlier series.8,9
Staphylococcus bacteriemia is more often associated with endocarditis than with pneumonia,5 so identification of this organism increases the need to rule out endocarditis, especially in patients with predisposing diseases or an intravascular catheter. Interestingly, fungi were rarely identified as the causal agent despite the fact that most of our patients had received previous antibiotic treatment, were immunosuppressed, or had an intravascular catheter. Streptococcus viridans was notably absent, as in an earlier study.8 Cases of right-sided endocarditis that remained undiagnosed because they were confused with respiratory infections (and improved with antibiotic treatment) were probably caused by nonaggressive germs such as streptococci. This would explain why Staphylococcus aureus was the most frequent microorganism in our series, as those infections caused by more aggressive germs are only diagnosed when the patient fails to improve with treatment for pneumonia.
Vegetations were a constant finding in our patients. Moreover, these formations were large as a result of the lower pressure exerted by the right heart chambers, which allows vegetations to grow larger than on the left side.22 Many studies have associated large, mobile vegetations with a high incidence of embolisms,23.24 which may explain the frequent presence of pulmonary embolisms in our series.
In most of our patients the clinical course was satisfactory with medical treatment alone. Surgery was needed in 29% of the patients, a proportion lower than in earlier series8 probably because of improvements in antibiotic treatment in recent decades. The reasons for surgery were similar to those for right-sided endocarditis in general.1,5,8,9 All surgical patients had a satisfactory clinical course. In contrast to the situation in patients with IVDU, reinfection of the prosthesis is not a frequent problem in non-IVDU patients.2 Only one of our patients relapsed, after 3 months. Mortality related with the disease was low, and lower than the mortality associated with left-sided endocarditis, although the comparison did not reach statistical significance probably related to the difference in the sample size between both groups.
CONCLUSIONS
In conclusion, our results indicate that isolated right-sided endocarditis should be included in the differential diagnosis in patients with febrile syndrome, respiratory symptoms and predisposing disease (cancer, immunodepression, or chronic renal failure), even if they do not have a pacemaker and have no history of IVDU. The presence of an intravascular catheter, recent surgical procedures and staphylococcus bacteriemia should reinforce the suspicion of right-sided endocarditis. The prognosis appears to be more favorable than for left-sided endocarditis.
This study was financed in part by the Cooperative Network for Cardiovascular Research (Red Cooperativa de Investigación Cardiovascular, RECAVA) of the Spanish National Institute of Health (Instituto de Salud Carlos III) and by a grant from the Sociedad Castellano-Leonesa de Cardiología (SOCALEC).
ABBREVIATIONS
IVDU: intravenous drug use
Correspondence: Dra. A. Revilla Orodea.
Departamento de Cardiología y Cirugía Cardiaca. Instituto de Ciencias del Corazón (ICICOR). Hospital Clínico Universitario.
Ramón y Cajal, 3-5. Valladolid. España.
E-mail: arevillaorodea@gmail.com
Received April 15, 2008.
Accepted for publication September 16, 2008.