Keywords
INTRODUCTION
Cardiac resynchronization therapy (CRT) represents one of the main advances made in recent years in the treatment of heart failure. The benefits obtained, both in terms of patient functional status and quality of life, and in the reduced number of hospitalizations required, are consistently reported in clinical trials.1-4 More recently, 2 large, multi-center trials5,6 have shown CRT to be associated with reduced long-term total mortality.
However, in all studies there are some 20%-30% of patients who do not improve with biventricular pacing,1,2 the reason for which is complex and multifactorial.7 The identification of clinical predictors8-10 of response is now growing given the unreliability of echocardiographic indices of ventricular asynchrony.11-13
Of basic importance in the analysis of response to CRT is the adequate placement of the left ventricular (LV) electrode. Normally, the electrode is implanted in the lateral region of the ventricle—the area where electrical activation arrives latest in a high percentage of patients with left bundle branch block (LBBB).14 However, while in idiopathic dilated cardiomyopathy the pattern is more predictable, in ventricular dysfunction of ischemic etiology the presence of areas of necrosis determines more variable patterns of activation.15-18 Patients with an abnormal intraventricular condition but with a normal QRS axis can show a ventricular activation pattern different to that seen in patients with a QRS axis deviated to the left in the presence of cardiomyopathy involving severe depression of LV systolic function.
The aim of the present work was to analyze the relationship between the QRS axis orientation and the response to CRT with respect to the location of the LV electrode.
METHODS
Inclusion Criteria
This prospective, observational study involved 80 consecutive patients referred to the electrophysiology laboratory at our center for CRT between August 2001 and June 2006. All patients fell into New York Heart Association (NYHA) functional class III/IV under optimum treatment for heart failure, and showed reduced LV systolic function (ejection fraction [EF] <35%) and a QRS complex time of >120 ms.
Implantation and Device Programming
The LV electrode was implanted with the aid of coronary sinus angiography, the target site being the lateral or posterolateral vein, as long as acceptable threshold stimulation was registered in the absence of phrenic stimulation. When these regions were inaccessible the electrode was implanted in the anterior interventricular vein. The device was programmed in DDDR stimulation mode in patients with sinus rhythm, and in VVIR mode in those with atrial fibrillation. In these latter patients the aim was to guarantee the greatest biventricular pacing possible, whether via drugs or via atrioventricular node ablation. Following implantation, the atrioventricular and ventriculoventricular intervals were optimized individually, guided by the echocardiographic assessment of LV asynchrony.
Follow-up and Definitions
All patients underwent a complete assessment at 6 and 12 months following device implantation. Functional class was determined by a clinical cardiologist independent of the clinician who implanted the device. Responders were defined as those patients who improved by at least one functional class, who also experienced at least a 5% increase in the left ventricular ejection fraction (LVEF), who required no hospitalization for heart failure, and who were still alive at 12 months. Failure to meet any one of these criteria led to the patient being defined a non-responder.
Patient electrocardiographic characteristics, QRS duration, and the electrical axis orientation were determined by a 12-lead electrocardiogram (ECG; 50 mm/s) immediately before and after the implantation of the resynchronization device. Patients were classified into 2 groups according to their preimplantation QRS axis orientation: those with a normal axis (from -30° to +120°), and those with a left axis (from -30° and -90°). Axis orientation was determined automatically but was manually confirmed by a cardiologist. The patients were also classified into 3 groups according to the location of the LV electrode: anterior (anterior interventricular vein or collateral veins), lateral (marginal or lateral vein), and posterior (posterolateral or middle cardiac vein). For the sake of precision, the anatomical location of the electrode was described from the left oblique anterior radiological position, where the tributary veins of the coronary sinus radiate like watch hands; the electrode was thus defined as lying between 1 and 5 o'clock.19
Echocardiographic data were obtained using a conventional Sequoia C 256 apparatus (Siemens AG, Munich, Germany). The EF was determined in the apical 4-chamber plane following the method of Simpson. The severity of mitral regurgitation was determined as the ratio between the maximum area of the regurgitation flow in color Doppler testing and the area of the left atrium. Patients were classified as having mild (ratio <20%), moderate (20%-40%) or severe (>40%) mitral regurgitation. The intraobserver and interobserver coefficients of variation with respect to EF measurements at our center are 3.5% and 4.4% respectively.
Statistical Analysis
Qualitative variables were expressed as numbers and percentages; quantitative variables were expressed as means (standard deviation). Differences between groups were analyzed using the Fisher-c2 test in the case of qualitative variables, and the Student t test or the Mann-Whitney test in the case of continuous variables. Logistic regression was used to identify the predictors of a good CRT response. The variables introduced into the model were age, etiology, preimplantation QRS axis, preimplantation EF, mitral regurgitation, location of the electrode in the coronary sinus vein, and the interaction between the electrode location and QRS axis orientation. Odds ratios (OR) and 95% confidence intervals (95% CI) were calculated from the regression coefficients obtained. A P value less than .05 was considered significant.
RESULTS
The percutaneous approach failed in 2 (2.5%) of the 80 patients referred for CRT. Four patients (5.1%) died during the follow-up year. Fifty-two patients (66.7%) responded to CRT. Table 1 shows the baseline characteristics of the 78 surviving patients. The mean age was 70 (7) years; 57 patients (73%) were male. Sixty seven (86%) patients fell into NYHA functional class III; 11 (14%) fell into functional class IV. The mean preimplantation duration of QRS was 172 (23) ms; the mean preimplantation LVEF was 27% (7%). Ischemia was the cause of cardiomyopathy in 31 (40%) patients. Atrial fibrillation was seen in 23 (29%) patients. The atrioventricular node was ablated in 9 (39%) patients with atrial fibrillation to guarantee biventricular pacing. No significant differences were seen in baseline clinical characteristics with respect to QRS axis orientation (Table 2).
The LV electrode was implanted in the lateral vein in 27 (35%) patients; in 18 (23%) it was placed in a posterior vein, and in 33 (42%) it was implanted in the anterior interventricular vein. No significant difference was seen in the location of the LV electrode with respect to QRS axis orientation (Table 2). All the electrodes in the right ventricle were located in the apex.
Table 3 shows the relationship between baseline clinical characteristics and the response to CRT. This response was somewhat better in the group of patients with left axis deviation (72.2%) than in the patients with normal axis (62.2%), although this difference was not significant (P=.331). The preimplantation EF was lower in the responders (25.7% [5.3%]) than in the non-responders (28.7% [5.5%]) (P=.023). The response was also better in patients with mitral regurgitation (73.1%) than in those without this condition (46.2%) (P=.019). No significant differences were seen in CRT response with respect to NYHA functional class, ischemic or idiopathic etiology, heart rhythm, the reduction of the QRS width, conduction abnormalities or the vein in which the LV electrode was implanted.
The anatomical position of the LV electrode implanted in the anterior interventricular vein in left oblique anterior radiological projection was: 23 (70%) at 1 o'clock; 8 (24%) at 2 o'clock, and 2 (6%) at 3 o'clock. Therefore, 30% of these electrodes were in a upper and lateral position. In the lateral vein 22 (81%) were at 3 o'clock, 4 (15%) were at 4 o'clock, and 1 (4%) at 2 o'clock. In the posterior vein, 15 (83%) were at 4 o'clock, 2 (11%) were at 3 o'clock, and 1 (6%) was at 5 o'clock. Thus, the majority of electrodes implanted in a posterior vein were in a lower position.
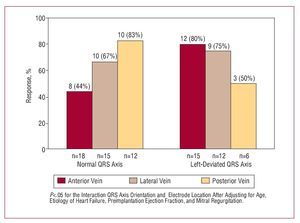
With respect to electrode implantation in the anterior interventricular vein, more responders were seen among the patients with a left axis deviation: 12 (80%) patients with such an axis orientation responded to CRT, compared to 8 (44.4%) of those with a normal QRS axis (P=.037). With respect to electrode implantation in the lateral vein, no significant differences were seen in CRT response between patients with a left-deviated or normal QRS axis (75% compared to 66.7%). With respect to electrode implantation in the posterior vein, no significant differences were seen in CRT response between patients with a left-deviated or normal axis QRS either (50% compared to 83.3%) (Figure).
Figure. Response to resynchronization therapy with respect to QRS axis orientation and location of the LV electrode.
Logistic regression adjusted for age showed the response to CRT to be associated with a left axis (OR=5.04 [95% CI, 1-29.2]; P=.050), low EF (OR=0.91 [95% CI, 0.84-0.99]; P=.033), mitral regurgitation (OR=3.45 [95% CI, 1.13-10.6]; P=.030) and idiopathic etiology (OR=1.86 [95% CI, 0.61-5.72]; P=.278) (Table 4). A significant effect was also recorded for the interaction QRS axis orientation and electrode location (P=.026).
DISCUSSION
The most important finding of this study is that the QRS axis orientation (an ECG variable that can be simply and reproducibly measured) is a predictor of response to CRT. Importantly, the interaction of this variable with the location of the LV electrode also has a significant effect on response; patients in whom the electrode is implanted in the anterior interventricular vein show a better response if their preimplantation QRS axis is left-deviated. These data may be clinically important, especially with respect to the selection of patients with heart failure for CRT, and in the optimization of the location of the LV electrode.
The patients with a left-deviated QRS axis showed a more favorable response to CRT than those with a normal QRS axis. This better prognosis was observed after adjusting for patient age and other variables that influence the response to therapy. However, this result may in part be due to the high percentage of electrodes implanted in the anterior vein (42%); it is in this position that patients with a left-deviated QRS axis showed a higher response rate than those with a normal QRS axis.
The identification of predictors of response to CRT, however, requires further investigation. To date, potential echocardiographic and clinical predictors have been investigated.7-9,20-26 The results of the PROSPECT study, which highlight the great variability between observers in terms of the echocardiographic variables used to assess left intraventricular mechanical asynchrony, have served to refocus attention on clinical variables as markers of possible response to CRT.13 Variables of interest mentioned in the literature include the etiology of heart failure,5-6,27 the duration of the preimplantation QRS,8,20-26 QRS reduction,8,20-26 and preimplantation brain natriuretic peptide levels,28 among others. However, the literature contains no reference to the importance of the preimplantation orientation of the QRS axis. All that has been described is the evidence for electrical remodeling in the native conduction system induced by resynchronization, characterized by a reduction in the QRS and a left deviation of the QRS axis.29 These variations probably reflect changes in the conduction system and the intramyocardial transmission of the cardiac impulse, and are the consequence of the chronic action of biventricular pacing.
Ischemic etiology has been described an independent factor associated with a lack of clinical response to CRT. This poorer response appears to be owed to the presence of scarring or areas of low perfusion that do not respond to stimulation.8 However, the large, clinical CARE HF6 and COMPANION5 studies showed the benefit of CRT to be independent of heart disease etiology. Further, and more recently, Vidal et al27 found no differences in clinical response nor in the degree of inverse ventricular remodeling, between patients whose disease was of ischemic etiology and those whose problems were of idiopathic dilated etiology. Certainly, no association was found in the present work between the QRS axis orientation and disease etiology. Indeed, the percentage of ischemic patients was greater in the left-deviated axis groups of patients (42%) than in the normal axis group (38%), which rules out etiology as a potential confounding factor in the relationship between response to CRT and QRS axis orientation.
Variable results have been reported with regard to the use of the preimplantation QRS duration or the reduction of QRS duration with biventricular pacing as predictors of the response to CRT.8,9,20-26,30 Clinical benefits and improved LV function have even been described in patients with heart failure, a normal QRS duration and echocardiographic evidence of left intraventricular and interventricular asynchrony.31 The present results show no significant differences in clinical response with respect to the duration of the baseline QRS. A greater reduction in the QRS with biventricular pacing has been reported in responders,8 but it has not been possible to establish the cut-off point that distinguishes between which patients will respond and which will not. In any event, this value will probably have to be adjusted for the baseline QRS value since reductions of the same magnitude may be associated with different responses depending on the width of the preimplantation QRS. In the present work, no significant differences were seen in clinical response with respect to QRS reduction, although this reduction was greater in responders. Although not statistically significant, the reduction in the QRS was greater in the left-deviated axis patients, which might indicate this to be a possible mechanism via which such patients achieve a better response to CRT.
The association between the location of LV stimulation and clinical response is a matter of controversy.8,24 It is currently accepted that the LV electrode should be positioned in the free wall since the lateral and posterolateral veins drain this area.17,18 In the present work, univariate analysis showed no association between the stimulation site in the coronary sinus vein and clinical response. However, after stratifying the results in terms of QRS axis orientation, better results were seen for location in the anterior or lateral veins in patients with a left-deviated QRS axis. In addition, with respect to location in the posterior vein, better results were seen in patients with a normal QRS axis (P=.026). This may be due to the different pattern of ventricular activation that occurs depending on the QRS axis orientation in patients with intraventricular conduction abnormalities and severe LV systolic dysfunction. Although no significant differences were seen in preimplantation QRS width between the groups, it may be that a greater delay in activation occurs in the upper and lateral regions of the left ventricle in patients with a left-deviated QRS. Therefore, a better response to CRT might be obtained if the electrode is implanted in the anterior interventricular vein, the position of which is also superior. This might be particularly true if the electrode is implanted in a collateral of the anterior interventricular vein, which allows for a more lateral placement. In the present work, 30% of the electrodes implanted in the anterior interventricular vein were in a superior and lateral position, which probably allowed for better resynchronization between the basal and apical segments. Such resynchronization is the basis of biventricular pacing at 3 sites, in which 2 electrodes are implanted in the LV.32
We believe these findings to be of interest, particularly the significance of the mentioned interaction. Further work is needed to confirm these findings in studies with a greater number of patients in different environments.
Limitations
The definition of clinical response to CRT was established as an improvement in functional class in the absence of a need for hospitalization owing to heart failure within the 12 months of the study period. Therefore, no 6 minute walking test was performed nor was the maximum consumption of oxygen determined.
The study included patients with atrial fibrillation, and the atrioventricular node was ablated in those who showed high ventricular frequencies (39%). Thus, these patients may have improved as a consequence of both therapies.
Since this was an observational study in which the location of the electrodes was not randomized, there may be other variables that influence the effect of the location of the electrode on the response to CRT.
CONCLUSIONS
The interaction QRS axis orientation and electrode location had a significant effect on the response to CRT. Patients in whom the electrode was implanted in the anterior interventricular vein responded better if they had a left-deviated QRS axis. With respect to the normal strategy of implantation, patients in whom the electrode cannot be placed in the lateral or posterolateral vein may be particularly benefited if the electrode is implanted in the anterior vein—if their preimplantation QRS axis is deviated to the left.
ABBREVIATIONS
CRT: cardiac resynchronization therapy
ECG: electrocardiogram
EF: ejection fraction
LV: left ventricle
SEE EDITORIAL ON PAGES 1236-8
Correspondence: Dr. F.J. García Seara.
Mónaco, 21, 2.O A. 15703 Santiago de Compostela. A Coruña. España.
E-mail: javiergarciaseara@yahoo.es
Received February 26, 2008.
Accepted for publication July 2, 2008.