Microvascular obstruction exerts deleterious effects after myocardial infarction. To elucidate the role of ischemia-reperfusion injury on the occurrence and dynamics of microvascular obstruction, we performed a preliminary methodological study to accurately define this process in an in vivo model.
MethodsMyocardial infarction was induced in swine by means of 90-min of occlusion of the mid left anterior descending coronary artery using angioplasty balloons. Intracoronary infusion of thioflavin-S was applied and compared with traditional intra-aortic or intraventricular instillation. The left anterior descending coronary artery perfused area and microvascular obstruction were quantified in groups with no reperfusion (thioflavin-S administered through the lumen of an inflated over-the-wire balloon) and with 1-min, 1-week, and 1-month reperfusion (thioflavin-S administered from the intracoronary catheter after balloon deflation).
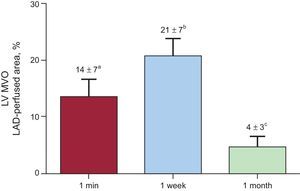
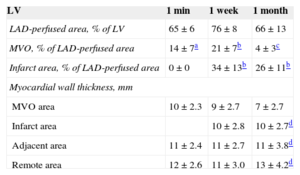
ResultsIn comparison with intra-aortic and intraventricular administration, intracoronary infusion of thioflavin-S permitted a much clearer assessment of the left anterior descending coronary artery perfused area and of microvascular obstruction. Ischemia-reperfusion injury exerted a decisive role on the occurrence and dynamics of microvascular obstruction. The no-reperfusion group displayed completely preserved perfusion. With the same duration of coronary occlusion, microvascular obstruction was already detected in the 1-min reperfusion group (14%±7%), peaked in the 1-week reperfusion group (21%±7%), and significantly decreased in the 1-month reperfusion group (4%±3%; P<.001).
ConclusionsWe present proof-of-concept evidence on the crucial role of ischemia-reperfusion injury on the occurrence and dynamics of microvascular obstruction. The described porcine model using intracoronary injection of thioflavin-S permits accurate characterization of microvascular obstruction after myocardial infarction.
Keywords
Timely and complete restoration of infarct vessel patency is the main goal in patients with acute myocardial infarction (AMI).1 Nevertheless, this approach does not ensure adequate reperfusion at the microvascular level, and impairment of perfusion persists in a significant number of patients.2 This phenomenon is referred to as microvascular obstruction (MVO) and exerts a strong negative impact after AMI.3–5
Ischemia-reperfusion injury has been extensively discussed in AMI6,7 and it could exert deleterious effects on microvascular integrity.2,3 Nevertheless, there is no definitive evidence demonstrating a direct association between reperfusion injury and the occurrence of MVO in myocardial samples obtained immediately after coronary reflow. Accurate in vivo animal models mimicking the dynamics of MVO in humans are urgently needed. Such models would permit a better understanding of the pathophysiology and timing of this process and, in turn, the exploration of new therapeutic opportunities under controlled conditions.
In the present study, we aimed to contribute proof-of-concept evidence on the crucial role exerted by ischemia-reperfusion injury on the occurrence of MVO and on the dynamics of this process. Up to now, contrasts used for studying perfusion in myocardial samples obtained from in vivo animal models have been infused in the left atrium,8 in the left ventricle (LV)9 or intravenously.10 To effectively study MVO, we performed a preliminary methodological study, which consisted of investigating the best route to administer thioflavin-S (T-S) to accurately define MVO.
METHODSExperimental StudyThirty-one juvenile domestic pigs weighing 25 kg to 30kg were used. The study protocol was approved by the local animal care and use committee and conforms to the current Spanish regulations (Royal Decree 53/2013, of February 1) and European Directive 2010/63/EC.
Further details on our study protocol can be consulted elsewhere.11,12 In summary, pigs were pretreated with intravenous amiodarone (300mg) and lidocaine (30mg) to reduce life-threatening arrhythmias. A 7 Fr sheath was introduced into the right femoral artery to monitor blood pressure and to access the left anterior descending coronary artery (LAD). A 7 Fr Amplatz Left 0.75 catheter was used to selectively engage the proximal LAD and a standard hydrophilic angioplasty wire was advanced and placed in the distal LAD. A 2.5mm x 15mm angioplasty balloon was inflated at 6 atm in the mid LAD distal to the first diagonal branch. Coronary artery occlusion was confirmed by contrast injection and by electrocardiographic ST-segment elevation.
Three groups of experiments with reperfusion were carried out. The balloon was deflated after 90min of coronary occlusion and restoration of normal coronary flow was documented by angiography. In the 1-min reperfusion group (n=5), 20mL of 4% T-S solution was selectively infused into the proximal LAD through the Amplatz Left 0.75 catheter 1min after balloon deflation, and hearts were arrested with potassium chloride and excised (Figure 1). Animals in the 1-week and 1-month reperfusion groups were allowed to recover and after 1 week (n=5) or 1 month (n=5) respectively, the same study protocol was followed and 20mL of 4% T-S solution was selectively infused into the proximal LAD through the Amplatz Left 0.75 catheter. Hearts were then arrested with potassium chloride and excised.
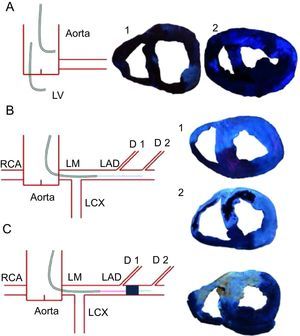
Methodology used for thioflavin-S infusion. Left, diagrams summarize the different methods used for thioflavin-S infusion. Right, pictures obtained under blue ultraviolet light. A: infusion in aorta (1) or in the left ventricle (2) resulted in a poor definition of the left anterior descending coronary artery-perfused area and microvascular obstruction. B: infusion into the catheter engaged in the proximal left anterior descending coronary artery resulted in a perfect definition of the left anterior descending coronary artery-perfused area and microvascular obstruction. This method was used in the control group (1) and in the 1-min, 1-week (2), and 1-month reperfusion groups. Light blue points represent the site of thioflavin-S infusion. C: infusion into the mid left anterior descending coronary artery through the lumen of an over-the-wire angioplasty balloon that was maintained inflated throughout the entire experiment resulted in a perfect definition of left anterior descending coronary artery-perfused area. This method was used in the no reperfusion group. D, diagonal; LAD, left anterior descending coronary artery; LCX, left circumflex artery; LM, left main stem; LV, left ventricle; RCA, right coronary artery.
Afterwards, to evaluate the role exerted by reperfusion injury on the occurrence of MVO, the 1-min reperfusion group was compared with a no-reperfusion group (n=5), which underwent an identical 90-min period of ischemia but without reperfusion. In this group of experiments, the balloon was not deflated and 20mL of 4% T-S solution was selectively infused into the mid LAD after the first diagonal branch through the lumen of an over-the-wire balloon (Figure 1). Immediately after T-S administration, hearts were arrested using potassium chloride and then excised.
The control group was made up of 5 experiments. In this group we used the same study protocol described above, but the angioplasty balloon was not inflated and thus ischemia and infarction were not provoked. We selectively infused 20mL of 4% T-S solution into the proximal LAD through the Amplatz Left 0.75 catheter. Hearts were then arrested with potassium chloride and excised.
A preliminary series of experiments was carried out to compare the transcatheter intraventricular and intra-aortic instillation with the methodology used in the present study (intracoronary infusion of T-S). The protocol described above was used to induce AMI in 6 pigs. Afterward, the angioplasty balloon was withdrawn and the pigs were allowed to recover. One week after infarction, the Amplatz Left 0.75 catheter was placed in the LV (n=3) or in the aorta (n=3), where 20mL of 4% T-S solution was infused. Hearts were then arrested with potassium chloride and excised. The precision of intra-aortic and intraventricular vs intracoronary infusion of T-S for assessing the LAD-perfused area and MVO was compared (Figure 1).
Furthermore, to assess the best method of T-S administration for discriminating the MVO area and the LAD-perfused area, the ratio of signal intensity between the MVO area and the LAD-perfused area negative for MVO was quantified in samples obtained from intraventricular, intra-aortic and intracoronary infusion using the software package MATLAB 6.5 (The Mathworks, Ink.; Nattick, Massachusetts, United States). This parameter was defined as the ratio between the signal obtained in LAD-perfused areas negative for MVO and the signal obtained in MVO areas. Higher values indicated better discrimination between these regions and, therefore, allowed better differentiation and quantification of MVO area.
Macroscopic Study of Myocardial SamplesImmediately after excision, the whole heart was viewed under ultraviolet light and photographed. Afterward, hearts were sectioned into 5-mm thick short-axis slices. To assess myocardial perfusion in the LV, each slice was viewed under ultraviolet light and photographed (Figure 1). The LAD-perfused area was defined as the percentage of the myocardial volume showing T-S staining. Dark blue areas were the zones not perfused by LAD whereas light blue areas were the LAD-perfused zones. Microvascular obstruction was interpreted as a lack of T-S staining in the core of the LAD-perfused area (Figure 2), and is expressed as the percentage of the LAD-perfused area.
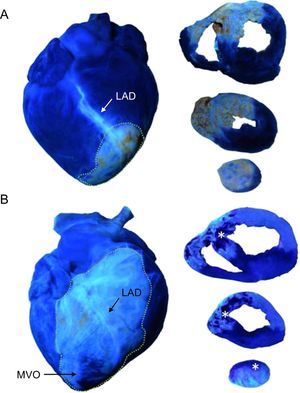
Effect of ischemia-reperfusion injury on the occurrence of microvascular obstruction. The images illustrate that under the same conditions and duration of coronary occlusion, perfusion was preserved in the no-reperfusion group whereas microvascular obstruction was detected as early as 1min after reperfusion. Asterisks indicate the microvascular obstruction area. Points delimit left anterior descending coronary artery-perfused area. A: example of an entire heart and slices of the no reperfusion group. B: example of an entire heart and slices of the 1-min reperfusion group. LAD, left anterior descending coronary artery; MVO, microvascular obstruction.
Thereafter, slices were incubated in 2,3,5-triphenyltetrazolium chloride 2% solution for 20min at 37 oC. Finally, they were viewed under room light and photographed. Infarcted tissue was defined as the myocardial area that did not stain with 2,3,5-triphenyltetrazolium chloride and is expressed as the percentage of the LAD-perfused area.
Myocardial wall thickness of the MVO, infarct, adjacent and remote areas was quantified in ultraviolet light images. The infarct area was defined as the area that did not stain with 2,3,5-triphenyltetrazolium chloride negative for MVO, the adjacent area was defined as the noninfarcted LAD-perfused area (with T-S and 2,3,5-triphenyltetrazolium chloride staining) and the remote area as the non-LAD-perfused myocardium (without T-S staining).
A separate subanalysis of the LAD-perfused area, MVO, infarcted tissue, and wall thickness of the right ventricle (RV) was performed using the same methodology as that for these parameters in the LV.
Images were digitalized and manual quantification of all short-axis slices was carried out offline in a dedicated laboratory (Cardiac Imaging Unit, INCLIVA, Valencia, Spain) by an experienced observer unaware of the protocol applied in each experiment. The software package MATLAB 6.5 was used. A ruler was photographed beside myocardial slices in all images and was used as a reference for measurements. This, along with the predefined slice thickness (5mm), permitted the calculation of LV and RV myocardial volumes.
Statistical AnalysisContinuous data are expressed as the mean±standard deviation and were compared by the unpaired Student's t test. Percentages were compared by the chi-square statistic; the Fisher exact test was used when appropriate. Statistical significance was considered for two-tailed P<.05. SPSS 22.0 (SPSS Inc.; Chicago, Illinois, United States) was used throughout.
RESULTSThree experiments were not completed: 2 due to refractory ventricular fibrillation and 1 due to technical problems in the radiology equipment. Electrical ventricular defibrillation was needed in 7 pigs during LAD occlusion and in 8 pigs during reperfusion. No significant complications were recorded over the reperfusion period.
Methodology Used for Thioflavin-S InfusionIntracoronary infusion of T-S, either from the catheter engaged in the proximal LAD (in the reperfusion groups) or from the lumen of the over-the wire balloon placed at the mid LAD (in the no-reperfusion group) resulted in much clearer definition of the LAD-perfused area and MVO than intraventricular or intra-aortic injection (Figure 1). According to these observations, the ratio of signal intensity (comparing the intensities of MVO area and LAD-perfused area negative for MVO) showed higher values in samples obtained after intracoronary infusion of T-S (5±1.9) than in samples obtained after intra-aortic (1±0.5) or intraventricular (1±0.3) infusion of T-S.
Dynamics of Microvascular ObstructionMicrovascular obstruction was detected in the LV myocardial samples from all reperfused experiments. Microvascular obstruction was detected as early as 1min after reperfusion, peaked at 1 week, and decreased at 1 month. The extent of MVO detected in the 1-month reperfusion group was significantly lower than that observed in the 1-week reperfusion group (Figure 3 and Table). Similar results regarding the dynamics of MVO occurred in the RV (Figure 1 and Table 1 of the supplementary material).
Dynamics of microvascular obstruction in the left ventricle. The extent of microvascular obstruction is represented as a percentage of the left anterior descending coronary artery-perfused area. Microvascular obstruction was already detected in 1-min group, peaked in the 1-week group, and partly resolved in the 1-month reperfusion group. LAD, left anterior descending coronary artery; LV, left ventricle; MVO, microvascular obstruction.
aP < .01 vs control group.
bP < .001 vs control group.
cP < .001 vs 1-week reperfusion group.
Left Anterior Descending Coronary Artery-perfused Area, Microvascular Obstruction, Infarct Area, and Myocardial Wall Thickness in the Left Ventricle in the 3 Series of Experiments
| LV | 1 min | 1 week | 1 month |
|---|---|---|---|
| LAD-perfused area, % of LV | 65±6 | 76±8 | 66±13 |
| MVO, % of LAD-perfused area | 14±7a | 21±7b | 4±3c |
| Infarct area, % of LAD-perfused area | 0±0 | 34±13b | 26±11b |
| Myocardial wall thickness, mm | |||
| MVO area | 10±2.3 | 9±2.7 | 7±2.7 |
| Infarct area | 10±2.8 | 10±2.7d | |
| Adjacent area | 11±2.4 | 11±2.7 | 11±3.8d |
| Remote area | 12±2.6 | 11±3.0 | 13±4.2d |
LAD, left anterior descending coronary artery; LV, left ventricle; MVO, microvascular obstruction.
In experiments performed under the same controlled conditions and duration of coronary occlusion, MVO only occurred in myocardial samples obtained from reperfused swine. Figure 2 depicts the crucial role of reperfusion injury on the occurrence of MVO. Whereas myocardial perfusion was completely preserved in myocardial samples obtained from all experiments in the no-reperfusion group, MVO was detected in all experiments in the 1-min reperfusion group.
Structural Consequences of Microvascular ObstructionInfarct tissue (as derived from 2,3,5-triphenyltetrazolium chloride staining) was detected in all cases in the 1-week and 1-month reperfusion groups (Table).
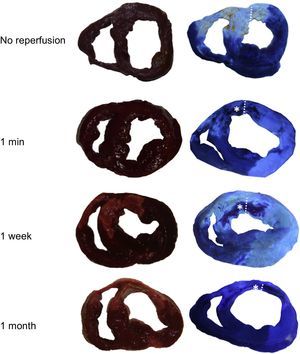
Left ventricle myocardial wall thinned in the MVO area in the 1-month reperfusion group compared with the infarct, adjacent and remote areas at the same time point (Table) and compared with the MVO area in the 1-week reperfusion group (Figure 4).
Consequences of microvascular obstruction on the left ventricular myocardial wall thickness. Slices of no reperfusion and 1-min, 1-week, and 1-month reperfusion groups stained with 2,3,5-triphenyltetrazolium chloride (left panels) and thioflavin-S (right panels). Asterisks indicate the microvascular obstruction area in the reperfused groups. Points delimit left ventricle wall thickness in the microvascular obstruction area. A significant thinning of the left ventricle wall in the microvascular obstruction area took place 1 month after reperfusion.
A similar tendency regarding the extent of the infarcted area and the association of MVO with progressive wall thinning occurred in the RV (Figure 2 and Table 1 of the supplementary material).
DISCUSSIONThe main contribution of the present study is the description of the crucial effect exerted by ischemia-reperfusion injury on the occurrence and dynamics of MVO. To perform a precise characterization of MVO in myocardial samples, an experimental model of anterior AMI based on intracoronary infusion of T-S was defined.
Methodology Used for Thioflavin-S InfusionAnimal models recreate human disease and constitute an essential tool for a better understanding of the underlying mechanisms. Currently, due to the similar size and cardiac physiology of swine and human heart, pigs represent the preferred specie for experimental studies not only in ischemia-reperfusion injury, but also in other cardiovascular diseases such as atherosclerosis.13–15 Although swine models of acute AMI have been well defined and widely used, methodology specifically focused on the characterization of MVO after AMI has not been developed.
Thioflavin-S, a dye that stains perfused endothelium, was used to quantitatively measure MVO. Intra-atrial,8 intraventricular9 and intravenous10 infusion of a variety of contrasts for different purposes such as for analysis of perfusion, area at risk, or MVO has been previously reported (Table 2 of the supplementary material). However, as far as we know, a specific methodological description of the contribution of intracoronary dye administration has not been explored. From our previous experience using intracoronary contrast echocardiography in humans, we learnt that this route offered the highest definition of myocardial perfusion.16 This background inspired us to undertake the present study. In the described swine model of anterior AMI, intracoronary infusion of T-S permitted excellent delineation of the LAD-perfused area and MVO in myocardial samples obtained immediately after sacrifice. Conversely, T-S infusion from intra-aortic and intraventricular routes resulted in a much poorer definition of myocardial perfusion. This observation was evident both by visual analysis of images and after quantification of the intensity ratio.
The intracoronary infusion of T-S was carried out either from the catheter engaged in the proximal LAD (once the angioplasty balloon had been deflated and removed) or through the lumen of an over-the wire balloon placed at the mid LAD (which was maintained inflated during the entire experiment). The first strategy was helpful to evaluate the dynamics of MVO at different time points of the ischemia-reperfusion process. The second model allowed us to explore the state of microvasculature after a long period of ischemia but immediately before the potentially deleterious effect exerted by reperfusion injury.
Thus, this novel strategy using intracoronary infusion of T-S appears to be a simple and reliable approach that permits accurate characterization of myocardial perfusion and MVO in myocardial samples obtained from an in vivo swine model of anterior AMI. This methodology may be helpful in the future to achieve further progress in understanding of the mechanisms underlying MVO and to explore the effects of new therapeutic opportunities addressed to prevent or reverse this process.
Dynamics of Microvascular ObstructionOver the early hours and days after reperfusion, changes occur in the state of microvasculature under the influence of multiple pathogenic components.2,3 We and others have demonstrated that, using imaging techniques in patients, there is a tendency toward spontaneous resolution of MVO during the weeks and months following reperfused AMI.2,3,17,18
The in vivo model presented here represents an ideal platform to characterize the dynamics of this process. For this purpose, we undertook experiments at different post-reperfusion times: 1min, 1 week, and 1 month after reperfusion. Important changes in the extent of MVO were observed: it was already detected immediately after reperfusion, reached its greatest extent at 1 week, and almost completely resolved at 1 month. Recent studies in a swine model have demonstrated other aspects of myocardial structural damage. They have observed that edema follows a bimodal pattern during the first week after AMI.19 Our results reveal that MVO also has a dynamic behavior characterized by an increment during the first week after AMI and a decrease at 1 month after AMI. These findings have potential implications in terms of diagnosis and therapy.
Firstly, there is no agreement regarding the most appropriate moment for evaluating MVO in patients with AMI. On the basis of our results, it could be suggested that analysis by imaging techniques before discharge (around 1 week after AMI) can provide an approximate estimation of the entire microvascular injury. Secondly, the dynamic behavior of MVO offers a therapeutic target beyond myocardial salvage. To date, apart from timely reperfusion within the very few first hours following coronary occlusion, approaches tested for reducing infarct size have been unsuccessful.20 Therefore, acting upon the damaged myocardium remains difficult. However, although the deleterious effects of a larger extent of MVO on patient outcome and LV remodeling have been well documented, so far the efficacy of therapies addressed to reduce MVO have failed and future endeavors must prove their value in addition to timely coronary reperfusion in rigorous randomized trials.21–23
As expected, infarcted tissue was not detected in controls, or in the no-reperfusion and 1-min reperfusion groups. The maximum extent of infarct area occurred 1 week after reperfusion. At 1 month, as a consequence of the shrinking process, infarct size diminished.
We have previously demonstrated that RV structural damage is not exclusive of inferior AMI. In anterior AMI, both infarct tissue and MVO can be detected in the anterior territory of the RV.11 The present study confirms the structural consequences of anterior AMI on the RV. The course of MVO in the RV paralleled that observed in the LV. This observation provides relevant information about the nature of MVO and reperfusion damage, but the clinical implications of these findings are uncertain.
Proof-of-concept Evidence of the Effect of Reperfusion on Microvascular ObstructionThe role exerted by ischemia-reperfusion injury in terms of myocardial damage has been largely debated. The pathogenesis of MVO is multifactorial and includes the combination of a variety of components.2,3 The significance of ischemia-reperfusion injury, if any, on this puzzle has not been totally understood. Controversy on the significance of ischemia-reperfusion injury has existed for years,6,7 but this mechanism has been suggested to play a role on MVO appearance.2,3
In this study, beyond theoretical statements, we aimed to contribute proof-of-concept data on the decisive influence of ischemia-reperfusion injury on MVO. To specifically address this issue, we designed 2 series of experiments in which anterior AMI was provoked using identical conditions. The only differing factor was reperfusion. In the first series, at the end of the 90-minute LAD occlusion, T-S was injected through the lumen of the balloon, which was maintained inflated; thus, reperfusion was not allowed. In the second group, at the end of the 90-min LAD occlusion, the balloon was deflated and removed and T-S was infused through the catheter engaged in the proximal LAD after a 1-min reperfusion period. Striking differences in terms of MVO were observed when myocardial samples of both series were compared. Whereas myocardial perfusion was completely preserved in the no-reperfusion group, MVO was observed in all cases that underwent as short as 1-min reperfusion.
In our view, the data presented contribute convincing evidence on the decisive role of ischemia-reperfusion injury on MVO. Currently, promising treatments against reperfusion injury exist but are still experimental.24 Furthermore, a number of medical or invasive approaches aimed at reducing MVO in reperfused AMI have been controversial or unsuccessful.21,22 Therefore, beyond the well-established effectiveness of timely primary percutaneous intervention, great efforts are still needed to better understand and manage the crucial peri-reperfusion minutes.
Study LimitationsFirst, the main limitation of the present study is the absence of a comparator technique to assess MVO in vivo, such as cardiac magnetic resonance imaging; a second technique confirming the results would have strengthened the conclusions obtained. Second, functional techniques evaluating the influence of MVO on cardiac function (such as strain by echocardiography or by intramyocardial crystals) could have provided some insight into the functional consequences of MVO, but these techniques are not available in our laboratory.
As always in basic research, translation to clinical practice needs caution. Data obtained in the present work could inspire studies in humans both to replicate our results using imaging techniques and to encourage the design of trials aimed at obtaining novel therapeutic tools to minimize the deleterious effect of reperfusion injury on microvascular perfusion.
CONCLUSIONSThe immediate onset of MVO following balloon deflation demonstrates the decisive role of ischemia-reperfusion injury on the occurrence of this process and demands awareness on the management of this short but critical time period in the revascularization of AMI patients. The in vivo experimental model presented could be helpful as a platform for further translational studies focused on a better understanding of MVO and on exploring alternative therapeutic opportunities under highly controlled conditions.
FUNDINGThe present study was supported by the Instituto de Salud Carlos III, the European Regional Development Fund (PI1400271 grant), and the Generalitat Valenciana (PROMETEO/2013/007 grant) and was awarded by the Reial Acadèmia de Medicina de la Comunitat Valenciana.
CONFLICTS OF INTERESTNone declared.