This article reviews the most relevant articles published in 2012 in the field of arrhythmias, on subjects that include clinical arrhythmology, ablation, cardiac pacing, and the genetics of sudden cardiac death.
Keywords
.
INTRODUCTIONThe branch of knowledge dealing with arrhythmias concerns clinical aspects, involving patients with cardiac rhythm disturbances, along with more specific aspects related to the drug or nondrug therapies for arrhythmias, whether the approach be ablation or cardiac pacing. These currently include the treatment of both bradyarrhythmias and tachyarrhythmias, as well as the management of patients with heart failure (HF).
In this article, we review the innovations reported in the literature related to the ablation of atrial fibrillation (AF) or ventricular tachycardia (VT), syncope, cardiac pacing, and the field of the genetics of sudden cardiac death.
ABLATION OF ATRIAL FIBRILLATIONOutcome and ComplicationsThe outcome and complications of ablation to treat AF continue to be a matter of debate.1 In a recent study, Calvo et al.2 analyzed the continuing improvements in outcome and complications in relation to optimization of the procedure and the learning curve. The study describes the experience in a single center and comprises 726 consecutive ablation procedures. The overall efficacy, including performance of more than 1 procedure in some patients, was 61%, with a mean follow-up period of 8 months. The authors divided the series into 2 groups, a first group (A), which consisted of 419 patients who underwent ablation prior to January 2008, and a second group (B), composed of 307 patients in whom ablation was performed after that date, accompanied by a strict anticoagulation and sedation protocol. They observed an improvement in the outcome and a reduction in the rate of major complications, which decreased from 6.2% in group A to 1.2% in group B. The independent predictors of successful ablation were type of AF (paroxysmal vs persistent), atrial anteroposterior diameter less than 45mm, and absence of hypertension and obstructive sleep apnea. We should point out that the rate of complications and the outcome can improve substantially with a favorable learning curve, the application of strict protocols, and careful patient selection. On the other hand, we should bear in mind that the assessment of the success of the procedure, measured as the time to the first documented recurrence, is a statistical goal that does not take into account the true clinical improvement experienced by the patient, which is probably greater.
New TechnologiesAmong the technical improvements that can facilitate the performance of AF ablation, one of the major advances is the introduction of rotational angiography (RA). Hadid et al.3 analyzed the utility of RA in the visualization of left atrium. To carry out this technique, adenosine was injected to induce a pause long enough to introduce the contrast material into left atrium. Analyzable images were acquired from 12 of 15 patients; 5 patients had been excluded previously for various reasons. The authors observed good agreement between these images and those obtained with magnetic resonance. Thus, RA could be used in place of other imaging techniques prior to the procedure, reducing costs and time. However, RA was performed with general anesthesia, which is not a customary practice in all the strategies employed in AF ablation.
Technology that makes it possible to measure the contact force applied to the distal electrode of the ablation catheter has recently been introduced in the procedure. The close relationship between the force exerted on the wall of the heart and the extension of the lesion is known on an experimental basis. However, the available data on the clinical utility is very limited. The results of the TOCATTA study have recently been published.4 The study was carried out in 32 patients and shows that all the patients in whom the mean force applied was less than 10g had recurrences, whereas 80% of the patients remained free from recurrence when the mean force was greater than 20g. The authors conclude that there is a good correlation between the contact force and the outcome of ablation and that, thus, this technical advance could improve the outcome significantly.
StrategiesSurgical ablation using a minimally invasive thoracoscopic technique may prove to be an alternative to AF ablation in patients with nonpermanent AF. The FAST study5 was the first to carry out a randomized comparison of catheter ablation and surgical pulmonary vein isolation in a group of patients who had recurrences after a first procedure or had dilated atria. In all, 124 patients from 2 centers were randomized. After a 12-month follow-up with 7-day Holter monitoring, only 36.5% of the patients treated with catheter ablation were totally free from recurrence, compared to 65.6% of the surgical patients. The latter group had a higher incidence of perioperative complications. The study indicates that the lesion achieved with surgery is more likely to be transmural and permanent, and thus more effective. However, given the higher rates of morbidity and mortality and the learning curve, at present this technique can be applied in very few centers.
Ablation of AF continues to be limited by the understanding of the underlying mechanism. While the prevailing view proposes the presence of multiple circulating wavelets, other authors defend more localized events, in which focal ablation would be possible. In the CONFIRM trial,6 the presence of focal mechanisms was analyzed using a complex mapping system, and the efficacy of mapping-guided ablation versus conventional ablation was compared. The investigators included 107 ablation procedures. They identified rotors or focal impulses in 97% of the cases. Ablation was guided by the mapping system in half of the patients. After a median follow-up period of 273 days, 82% of the patients who had undergone guided ablation were recurrence-free, compared to 45% of the patients treated with conventional ablation. The authors conclude that human AF is associated with rotors and focal mechanisms and that the ablation of these sources could improve the efficacy of the procedure.
VENTRICULAR TACHYCARDIAIn recent months, there has been an expansion of our knowledge in the field of VT ablation, which we will present in 3 sections.
New Procedures for Substrate AblationTwo recent studies refer to this aspect. The first compares a standard technique, involving targeted endocardial substrate ablation with induced VT, with a much more extensive endo-epicardial approach referred to as homogenization of the scar.7 This study included 92 patients with electrical storm: 49 were treated with substrate ablation limited to the region of the scar related to the induced VT and 43 underwent ablation of all the sites in the endocardium and epicardium showing abnormal electrograms. In this second group, regions of the epicardium in which the electrogram was abnormal were identified in 33% of the patients. In both groups, the ultimate aim was to suppress all the inducible VT, an objective that was achieved in every case. During a follow-up period of 25 (10) months, the rate of recurrences was significantly higher in the first group (47% vs 19%; P=.006). One patient in each group died during follow-up. This study demonstrates: a) that the suppression of inducibility is not the ultimate aim of ablation, and b) that the ultimate aim should be the elimination of the entire endocardial and epicardial substrate, even if its relationship to a specific tachycardia is not confirmed.
The second study was based on the hypothesis that eliminating the abnormal electrograms related to the scar and characterized by the presence of late potentials could be an effective and useful objective during substrate ablation.8 This study included 70 patients, 80% of whom had ischemic heart disease and 95.7% of whom had abnormal electrograms. During ablation, 70% of the electrographic abnormalities were abolished or dissociated. When the predictors of tachycardia recurrence or death during a 22-month follow-up were examined in a multivariate analysis, the elimination of all the abnormal electrograms was the only predictor (P=.02), whereas the lack of inducibility (P=.11) and the type of heart disease (P=.29) were not identified as predictors.
Assessment of the Objectives of the Ablation ProcedureAs was seen in the preceding section, there are many doubts as to whether the suppression of inducibility during programmed electrical stimulation is an ultimate aim of ablation.7,8 The next article we review provides data that explain the limitations of the key aims of ablation.9 The study assessed the value of noninvasive programmed stimulation some days after an ablation procedure. In a group of 132 patients, programmed stimulation with an implantable cardioverter-defibrillator (ICD) was undertaken at least 3 days after an ablation procedure. In this series, 44% of the patients had no inducible VT; in 37%, only nonclinical VT was induced; and 18% had inducible clinical VT. In all, 80%, 65%, and 30% of the patients, respectively, remained free of VT at 1 year. In the study performed immediately after ablation, clinical VT was induced in 6% of the patients, whereas in the subsequent study, it was induced in 18%. These findings indicate that the results of deferred programmed electrical stimulation have prognostic implications. Moreover, the second study is probably more sensitive with respect to the inducibility of clinical VT.
Alternatives to Radiofrequency AblationEpicardial CryoablationAlthough percutaneous radiofrequency (RF) ablation is safe and effective, this technique still cannot be employed to treat certain tachycardias, mainly those associated with nonischemic cardiomyopathy. Surgical ablation is an alternative, and the next study we analyze evaluates the utility of this technique as a complement to prior electrophysiological and electroanatomic characterization.10 In 8 patients with a previous electroanatomic study and unsuccessful RF ablation, surgical cryoablation was performed at the sites that had been identified and treated previously. During a mean follow-up period of 23 (6) months, 6 patients had a significant reduction in ICD shocks (from 6.6 to 0.6 shocks per patient; P=.026). The remaining 2 patients died, 1 of HF and the other from noncardiac causes. The study demonstrates that epicardial cryoablation applied to previously identified targets is effective in a majority of the patients who do not respond to RF ablation.
Transcoronary Ethanol AblationAnother of the alternative therapies that can be considered when conventional endo-epicardial ablation fails is transcoronary ethanol ablation, which is especially effective in cases in which the VT circuits are deep within the ventricular wall. In a series of 274 consecutive patients who underwent VT ablation, it was necessary to resort to transcoronary ablation in 27.11 The procedure was not completed in 5 patients because of unfavorable coronary anatomy. Following ablation, inducibility was suppressed in 82% of the patients. Although recurrence of VT was observed in 64% of the cases, of the 11 patients with electrical storm as the indication for ablation, only 2 experienced recurrence of this event.
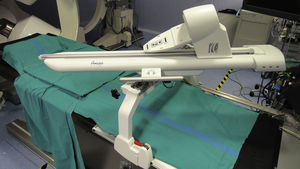
REMOTE NAVIGATIONA new generation of remote navigation systems was approved for clinical use in 2012. The Amigo™ Remote Catheter System, designed by Catheter Robotics, consists of a robotic arm that enables the remote manipulation of conventional ablation catheters (Fig. 1).12 It does not offer a priori any substantial advantage in the navigability or stability of the catheter when compared to conventional manual navigation because it employs the same catheter and is also based on mechanical force. However, the possibility of operating from outside the sterile field surrounding the patient and the fact that the operator can use other systems, such as the electroanatomic navigation system, at the same time facilitates the procedure and makes it go more smoothly. The system does not require a special location or infrastructure and is considerably less costly than the first-generation navigation systems. The other second-generation system is the CGCI® system designed by Magnetecs. This system is based on 8 electromagnets that, once the patient is properly positioned, enable the operator to guide a magnetic-tipped catheter by means of very rapid changes in the magnetic field in response to variations in the amount of electrical current flowing through each electromagnet (Fig. 2). The system is remotely controlled from a console with 2 joysticks, which enable highly intuitive navigation with practically no delays, unlike other magnetic remote navigators, like the Niobe® system, which are slower. This speed of response permits automatic navigation and ablation simply by marking target sites using a mouse placed on a 3-dimensional model of the heart cavity in a standard electroanatomic system. To date, excellent results with this system in an animal model13 and preliminary results in ablation of complex arrhythmic substrates in humans14,15 have been reported at congresses.
Robotic arm that supports the ablation catheter of the Amigo™ Remote Catheter System. The catheter handle is mounted on the arm and the distal part of the catheter is inserted via the femoral introducer. Movements are transmitted to the catheter through the robotic arm by means of a joystick located in a remote console.
In the meantime, clinical outcomes and improvements in first-generation remote navigation systems have continued to be presented. Some of the most notable improvements involve the Niobe® navigation system from Stereotaxis, which now incorporates the possibility of using a robotic arm that enables the remote manipulation of another catheter, specifically a circular catheter for pulmonary vein mapping, while continuing with the magnetic navigation of the ablation catheter.16 This is especially important for procedures such as the electrical isolation of the pulmonary veins for AF ablation, for which it is necessary to move the circular catheter from vein to vein as each is isolated.
Regarding the clinical outcome, results with the use of remote navigation systems for the ablation of complex arrhythmia substrates have been published. In this respect, the use of magnetic navigation systems for ablation in patients with complex congenital heart disease has been reported, with good results and advantages over conventional manual navigation in these cases of complex anatomy.17 A worldwide survey of the experience with AF ablation using the Sensei® robotic navigation system from Hansen Medical has also been presented. It showed that a learning curve of 50 cases is necessary to reduce the rate of complications in AF ablation compared to conventional ablation.18
Finally, a significantly higher efficacy has been reported for remote magnetic navigation in VT ablation, especially in patients without structural heart disease.19
SYNCOPEIn 2012 the most relevant issues with respect to syncope refer to therapeutic aspects regarding both the confirmation of the absence of effective drug therapy and the role of permanent cardiac pacing in some types of neurally mediated syncope.
Drug TherapyTwo articles have discussed at great length the inefficacy of drug therapy in patients with neurally mediated syncope. In recent years, based on controlled studies involving small numbers of patients, it was proposed that midodrine might be the only drug to be useful in the treatment of patients with recurrent neurally mediated syncope. Taking into account that in the majority of the publications that have studied the efficacy of midodrine, the drug has been evaluated as the treatment of choice in comparison with conventional measures, and given that nondrug treatment (such as counterpressure maneuvers) now appears to be clearly the first-line choice and, moreover, is effective in over 50% of the patients, Romme et al.20 performed a controlled crossover study comparing midodrine versus placebo in patients with recurrent vasovagal syncope in whom nondrug therapeutic measures had failed. In this group of patients, the authors found no beneficial effect of midodrine compared with placebo. This same group of investigators carried out a meta-analysis of 46 randomized studies in which they reviewed the effectiveness of different drugs in vasovagal syncope (40 studies) or carotid sinus syncope (6 studies), and concluded that the data available at the present time does not enable us to recommend the use of any drug therapy for vasovagal syncope or syncope secondary to carotid sinus syndrome.21
Cardiac Pacing in Neurally Mediated SyncopeIn early 2012, 2 studies were published that contribute to clarifying the role of cardiac pacing in patients with neurally mediated syncope and asystole. The first22 included 80 patients with unexplained syncope; after intravenous administration of 20mg of ATP (adenosine triphosphate), syncope lasting over 10s was observed. These patients underwent implantation of a dual-chamber pacemaker (make and model at the discretion of the investigator) and were randomized to DDD pacing at 70 bpm (active pacing) or AAI pacing at 30 bpm (inactive pacing or placebo). During a 5-year follow-up period, the recurrence rate was 21% in the patients with active pacing versus 66% in those with AAI pacing. On the other hand, the ISSUE-3 study23 included patients over 40 years of age with recurrent neurally mediated syncope in whom pauses of more than 3s during spontaneous syncope and more than 6s with or without symptoms had been documented by an implantable loop recorder. These patients underwent pacemaker implantation with a rate drop response algorithm and, following implantation, were randomized to DDD mode or inactive pacing. During a 2-year follow-up, the rate of recurrence was 57% in the unpaced group and 25% in the patients with active pacing.
These findings suggest that selected patients, with unexplained or neurally mediated syncope in whom asystole is documented, whether during a spontaneous syncopal episode or during syncope in response to the ATP test, may benefit from pacemaker implantation. In any case, this therapeutic approach should be reserved as a last resort in highly symptomatic patients, and should be avoided in young patients.
Syncope in Patients With Bundle Branch BlockThe approach in patients with syncopal episodes, bundle branch block, and no structural heart disease has yet to be fully elucidated. Whereas some authors propose pacemaker implantation in most of these patients, others propose a tiered strategy, consisting in first performing an electrophysiology study and then, if the results are normal, implantation of a loop recorder. Azocar et al.24 have published an article in which they use this strategy in a group of 85 patients with syncope and bundle branch block and report that the electrophysiology study was diagnostic in 36 (42%). Of the 38 patients in whom a loop recorder was implanted, 13 (34%) had some diagnostic finding. In general, the most common diagnosis was paroxysmal block, observed in 37 patients (43%), followed by VT in 6 (7%). During follow-up, there were 2 deaths (2.3%), both due to noncardiac causes. These findings were similar to those reported by Moya et al.25 and confirm that this is a safe strategy that makes it possible to establish the diagnosis in a relatively high percentage of patients.
CARDIAC PACINGPacemakersDuring 2012, 895 articles have been published with the term “cardiac pacing” as a keyword. Here we summarize the most relevant publications grouped according to subject.
Pacemakers in Transcatheter Aortic Valve ImplantationIn 2012, there have been 2 articles of special relevance concerning the reduced need for pacemaker implantation following transcatheter aortic valve implantation. In the first,26 a conservative strategy was applied in patients with complete left bundle branch block during the procedure to avoid cardiac pacing. The authors found no differences in 1-year mortality between the patients with left bundle branch block in whom pacemakers were not implanted and those without left bundle branch block.
The second article27 analyzed the factors that predict and affect the need for pacemaker implantation following transcatheter aortic valve implantation with the CoreValve prosthesis, using a new delivery system that theoretically is associated with less traumatic injury to the left ventricular (LV) outflow tract, reducing the probability of changes in atrioventricular conduction.
Selective Right Ventricular PacingA meta-analysis was published offering a systematic review of the randomized studies that compare the benefits of nonapical pacing with respect to apical pacing28; it concludes that, although patients with nonapical pacing had a higher left ventricular ejection fraction than patients with apical pacing, there were no differences in their exercise capacity, functional class, quality of life, or survival.
We also consider notable a review article by Hillock and Mond,29 which analyzes all aspects of right ventricular (RV) septal pacing.
Cardiac Pacing and Atrial FibrillationThe main objective of the EPASS study30 was to analyze the prevention of AF in patients with sinus node disease by means of pacing from right atrial appendage or from lower interatrial septum. It concluded that, in the subgroup of patients with sinus node disease and delayed intraatrial conduction time, pacing from lower interatrial septum is more effective in preventing the progression of AF.
The multicenter study ASSERT31 a multicenter study, analyzed the presence of clinical AF and the risk of stroke in 2580 patients with pacemakers or ICD and with no known history of AF. Asymptomatic AF was detected in 10% of the patients after 3 months of follow-up, an incidence that rose to 24% after 2.5 years of follow-up. The patients in whom subclinical atrial arrhythmias were detected had a higher incidence of clinical AF and of stroke or peripheral embolism over a mean follow-up period of 2.5 years.
Brignole et al.32 performed a randomized study in which they analyzed the clinical response to atrioventricular junction ablation and pacemaker implantation in patients with rapid, symptomatic, drug-refractory AF, comparing RV pacing with cardiac resynchronization therapy (CRT). The primary outcome measure was defined as the combination of death, hospital admission for HF, and worsening HF. The rate of responders, defined as the absence of these 3 events, was 63% in the right ventricular pacing group and 83% in the group with CRT,32 in which there were lower incidences of both hospital admissions due to HF and worsening HF, whereas no differences were observed in the mortality rate. CRT and optimization of CRT with echocardiography were the only indicators of clinical benefit.33
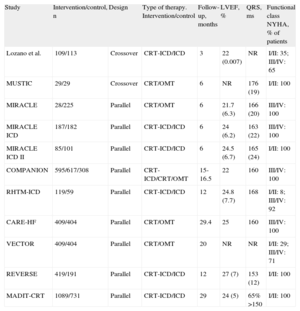
Cardiac Resynchronization TherapyCandidate SelectionThe utilization of CRT devices for the treatment of HF continues to increase.34 Three meta-analyses have evaluated the response of different groups of patients to CRT. Wells et al.35 analyzed 12 randomized studies (Table 1) in which the utility of associating CRT with optimal medical therapy or with an implantable cardioverter-defibrillator was investigated. They concluded that in patients with HF the implantation of a CRT device combined with optimal therapy or an ICD reduces mortality and that this benefit is especially evident in patients with moderate symptoms (New York Heart Association functional class II).
Studies Cited in the Meta-Analysis Performed by Wells et al.35
| Study | Intervention/control, n | Design | Type of therapy. Intervention/control | Follow-up, months | LVEF, % | QRS, ms | Functional class NYHA, % of patients |
| Lozano et al. | 109/113 | Crossover | CRT-ICD/ICD | 3 | 22 (0.007) | NR | I/II: 35; III/IV: 65 |
| MUSTIC | 29/29 | Crossover | CRT/OMT | 6 | NR | 176 (19) | I/II: 100 |
| MIRACLE | 28/225 | Parallel | CRT/OMT | 6 | 21.7 (6.3) | 166 (20) | III/IV: 100 |
| MIRACLE ICD | 187/182 | Parallel | CRT-ICD/ICD | 6 | 24 (6.2) | 163 (22) | III/IV: 100 |
| MIRACLE ICD II | 85/101 | Parallel | CRT-ICD/ICD | 6 | 24.5 (6.7) | 165 (24) | I/II: 100 |
| COMPANION | 595/617/308 | Parallel | CRT-ICD/CRT/OMT | 15-16.5 | 22 | 160 | III/IV: 100 |
| RHTM-ICD | 119/59 | Parallel | CRT-ICD/ICD | 12 | 24.8 (7.7) | 168 | I/II: 8; III/IV: 92 |
| CARE-HF | 409/404 | Parallel | CRT/OMT | 29.4 | 25 | 160 | III/IV: 100 |
| VECTOR | 409/404 | Parallel | CRT/OMT | 20 | NR | NR | I/II: 29; III/IV: 71 |
| REVERSE | 419/191 | Parallel | CRT-ICD/ICD | 12 | 27 (7) | 153 (12) | I/II: 100 |
| MADIT-CRT | 1089/731 | Parallel | CRT-ICD/ICD | 29 | 24 (5) | 65% >150 | I/II: 100 |
CRT, cardiac resynchronization therapy; ICD, implantable cardioverter-defibrillator; LVEF, left ventricular ejection fraction; NR, not reported; NYHA, New York Heart Association; OMT, optimal medical therapy.
Values are expressed as mean (standard deviation), unless otherwise indicated.
The results of the meta-analysis by Al-Majed et al.36 strongly support the finding that CRT is beneficial in patients with ventricular dysfunction and wide QRS, regardless of the severity of the symptoms.
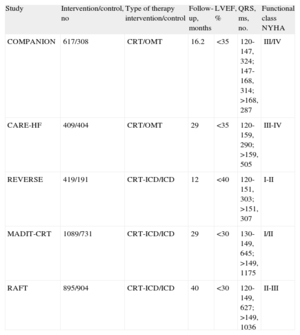
In the third meta-analysis, Sipahi et al.37 analyzed 5 studies (Table 2) to determine the importance of the duration of the QRS interval as a predictor of the utility of CRT, and concluded that resynchronization reduced the incidence of adverse clinical events in patients with HF and a QRS≥150ms.
Studies Cited in the Meta-Analysis by Sipahi et al.37
| Study | Intervention/control, no | Type of therapy intervention/control | Follow-up, months | LVEF, % | QRS, ms, no. | Functional class NYHA |
| COMPANION | 617/308 | CRT/OMT | 16.2 | <35 | 120-147, 324; 147-168, 314; >168, 287 | III/IV |
| CARE-HF | 409/404 | CRT/OMT | 29 | <35 | 120-159, 290; >159, 505 | III-IV |
| REVERSE | 419/191 | CRT-ICD/ICD | 12 | <40 | 120-151, 303; >151, 307 | I-II |
| MADIT-CRT | 1089/731 | CRT-ICD/ICD | 29 | <30 | 130-149, 645; >149, 1175 | I/II |
| RAFT | 895/904 | CRT-ICD/ICD | 40 | <30 | 120-149, 627; >149, 1036 | II-III |
CRT, cardiac resynchronization therapy; ICD, implantable cardioverter-defibrillator; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; OMT, optimal medical therapy.
On the basis of these studies, the Heart Failure Society of America has prepared an update of the guidelines,38 the ultimate conclusion of which can be summarized by saying that the current scientific evidence supports the use of CRT in patients with left ventricular dysfunction with mild to severe symptoms. This evidence becomes “convincing” in those with a QRS≥150ms if they do not have right bundle branch block.
Left Ventricular Pacing AloneThibault et al.39 published the results of a multicenter, double-blind, crossover study in which they compared the effects of left ventricular pacing alone on exercise tolerance and LV remodeling with those of biventricular pacing. The study included 211 patients with left ventricular ejection fraction≤35%, QRS≥120ms, and symptoms of HF. The authors concluded that left ventricular pacing alone is not superior to biventricular pacing; however, the patients who did not respond to biventricular pacing could respond to left ventricular pacing; thus, the nonresponders could be evaluated on the basis of the response to LV pacing alone.
Remote Follow-up in Cardiac PacingHome monitoring (HM) systems for use by patients with cardiac pacemakers are undergoing a series of structural changes that are arousing the interest of arrhythmology specialists. Nevertheless, acceptance of the introduction of this care strategy in Spain continues to vary widely. Thus, we consider it of interest to include the innovations in this field with the intention of providing impetus to its use in all specialized units.
A growing number of studies have shown HM to be reliable and safe, and it is well accepted by patients and professionals.40,41 Along these lines, the ALTITUDE study,42 a nonrandomized study of 194 006 patients with a 5-year follow-up period, showed an improvement in the survival of the patients that utilized HM compared to routine follow-up visits.
In 2012, a number of randomized studies have demonstrated that the use of HM reduces emergency department visits,43 scheduled visits for device follow-up,43–45 and the time elapsed between detection and solution of clinical events.44 In this respect, a consensus document46 has also been published that summarizes the current status of this technology, the direction it is heading, and what aspects remain to be addressed.
Finally, it should be mentioned that the implementation of HM is accompanied by the development of a group of electronic tools that facilitate the automatic collection and standardization of the information from the follow-up visits and remote monitoring, regardless of the manufacturer, which simplifies the use of the data for quality control and clinical research and their automatic inclusion in the electronic medical record. In Spain, nationwide initiatives to create multicenter databanks have surpassed all expectations in the inclusion of patients and proposals for clinical trials.
Electromagnetic Interference and Magnetic Resonance ImagingIn 2012, a detailed and updated review of electromagnetic interference with pacing and defibrillation devices in- and outside of the medical environment was published in 2 consecutive articles.47,48
Nazarian et al.,49 with an excellently designed prospective study, concluded that with adequate precaution, patients with pacemakers and ICD can undergo magnetic resonance imaging with very little risk.
At present, pacemakers are available that are compatible with magnetic resonance and have been approved for clinical use. However, we should point out that the use of these devices continues to be subject to their adjustment and examination prior to, during, and after the performance of this imaging study. Thus, it is important to know under what conditions they should be used.50
GENETICS OF SUDDEN CARDIAC DEATHThe most relevant topic in 2012 is the confirmation of titin as a gene associated with dilated cardiomyopathy,51 hypertrophic cardiomyopathy,51 and arrhythmogenic RV cardiomyopathy.52TTN, the gene that encodes titin, has not been extensively studied due to the size of its coding region (it is the largest human protein). Its association with dilated cardiomyopathy was reported in 2002.53 However, studies of the prevalence of mutations in this gene in cardiomyopathies have not been performed.
In 2011, 27 families with arrhythmogenic right ventricular dysplasia were evaluated and TTN mutations were found in 7 families, with clear segregation of the mutation among those family members that were affected.52 This study confirmed the association between sarcomeric proteins and dysplasia, a circumstance that creates a new overlap in the genotype and the phenotype of cardiomyopathies. Thanks to the development of next-generation sequencing, the authors of a recent study analyzed 312 patients with dilated cardiomyopathy and 231 with hypertrophic cardiomyopathy. They identified a prevalence of TTN truncating mutations in 25% of the patients with dilated cardiomyopathy and in 18% of the patients with a sporadic form of the disease. The prevalence of mutations in hypertrophic cardiomyopathy was 1%.
These 2 studies confirm the association between the largest human protein and cardiomyopathy. Genetic studies are beginning to incorporate next-generation sequencing, a very powerful technology that permits the simultaneous analysis of hundreds of genes. This technology will enable us to advance rapidly in genetic research and diagnosis. However, the amount of data obtained undoubtedly increases the complexity of genetic studies and intensifies the need to include quality genetic studies with a highly specialized clinical evaluation.
CONFLICTS OF INTERESTNone declared.