Our aim was to assess the impact of prosthetic pulmonary valve replacement (PVR) in patients with repaired tetralogy of Fallot (rTOF) on changes in biventricular volumes and function and on adverse cardiac events.
MethodsAdults with rTOF were identified from the SACHER-registry. Data from serial cardiac magnetic resonance imaging, echocardiography, exercise capacity and n-terminal pro b-type natriuretic peptide (NT-proBNP) were collected. The primary endpoint was right ventricular ejection fraction (RVEF) as measured by cardiac magnetic resonance. Secondary endpoints were biventricular volumes, left ventricular ejection fraction, exercise capacity and NT-proBNP levels, and time to adverse cardiac outcomes (atrial and ventricular arrhythmia, endocarditis). Associations between previous PVR and longitudinal changes in functional outcomes and time to adverse cardiac outcomes were analyzed using linear mixed-effects models and Cox proportional hazards models, respectively.
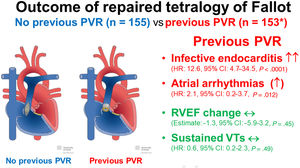
ResultsA total of 308 patients (153 with and 155 without PVR) with 887 study visits were analyzed. Previous PVR was not significantly associated with changes in RVEF (CE, −1.33; 95%CI, −5.87 to 3.21; P=.566). Previous PVR was associated with lower right ventricular end-diastolic volume but had no significant effect on left ventricular ejection fraction, exercise capacity, or NT-proBNP-levels. Previous PVR was associated with an increased hazard of atrial arrhythmias (HR, 2.09; 95%CI, 1.17-3.72; P=.012) and infective endocarditis (HR, 12.72; 95%CI, 4.69-34.49; P<.0001) but not with an increased hazard of sustained ventricular arrhythmias (HR, 0.64; 95%CI, 0.18-2.27; P=.490).
ConclusionsPrevious PVR was not significantly associated with changes in RVEF but was associated with an increased risk of atrial arrhythmias and infective endocarditis.
Keywords
Based on associations found in retrospective studies, pulmonary regurgitation (PR) has been postulated to be an important risk factor for late onset of arrhythmic complications and progressive right ventricular systolic dysfunction in patients with repaired tetralogy of Fallot (rTOF).1 Based on these assumptions, prosthetic pulmonary valve replacement (PVR) in patients with moderate to severe PR (even in the absence of symptoms) has been advocated to preserve right ventricular systolic function and reduce long-term complications.1–3 Recently, the INDICATOR cohort, a prospective, well defined, international cohort study of >800 patients with rTOF provided important new insights into risk stratification and the role of PVR for risk reduction in patients with rTOF.4 Data from the INDICATOR cohort identified right ventricular ejection fraction (RVEF), as measured by cardiac magnetic resonance imaging (CMR), as an independent predictor of arrhythmic complications, while right ventricular volumes and the severity of PR were not associated with adverse events.5 While a first propensity score-adjusted analysis of the INDICATOR cohort failed to demonstrate a prognostic benefit of PVR, its most recent analysis with longer follow-up demonstrated a lower risk of the composite endpoint of death or sustained ventricular tachycardia after PVR.6,7
The aims of our study were therefore to investigate the associations of PVR with long-term changes in right and left ventricular systolic function, and with the occurrence of long-term cardiovascular complications.
MATERIAL AND METHODSThis was a retrospective cohort study. For reporting, we followed the recommendations of the STROBE guideline.8 We identified adults with rTOF followed up at 3 tertiary care Swiss centers, previously enrolled in a Swiss registry (SACHER-registry). The detailed methods and protocols of this registry have already been published.9 The registry and the current data analyses were approved by the local ethics committees and all patients provided written informed consent for analysis of clinical data at the time of enrolment in the registry. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki.
Patient cohortThe study cohort consisted of patients identified in the SACHER-registry with rTOF who were followed up at one of the participating centers. We excluded patients with pulmonary atresia variants, requiring primary repair with prosthetic conduits, and patients without CMR or quantitative echocardiography studies.
Data collectionDemographic characteristics included age at intracardiac repair, information related to palliation (procedures prior to intracardiac repair) and PVR status, as well as age at palliation and/or PVR, if performed. Data on date of birth, age at intracardiac repair, age at first visit, palliation status and age at palliation were directly extracted from the SACHER-registry. Data collection related to PVR, CMR, echocardiography, electrocardiography (ECG) n-terminal pro-brain natriuretic peptide (NT-proBNP), exercise capacity and clinical history was performed by chart review. For the purpose of this study, we defined the first visit as the clinical visit when the first CMR study was performed. To assess changes in ventricular volumes and systolic function, exercise capacity and NT-proBNP, data from clinical reports during follow-up visits were recorded and analyzed. All study investigations were performed during routine clinical follow-up, following guideline recommendations.10–12
Data retrieved from CMR study reports included right and left ventricular end-diastolic volumes, normalized to body surface area (RVEDVi and LVEDVi, respectively), right and left ventricular ejection fraction (RVEF and LVEF, respectively) and PR fraction in percent.
Data retrieved from echocardiography reports included the severity of PR (none, mild, moderate, severe), right ventricular end-diastolic area on 4-chamber view, fractional area change of the right ventricle on 4-chamber view, tricuspid annular plane systolic excursion (TAPSE), tricuspid annular systolic velocity, and LVEF. Peak systolic pressure gradients across the right ventricular outflow tract (as a measure of right ventricular outflow tract obstruction) and across the tricuspid valve (as a measure of right ventricular systolic pressure) were recorded.
Data retrieved from reports of cardiopulmonary exercise testing included peak workload (peak WL) and maximal O2 consumption (peak VO2) and its percentages of predicted values, as well as maximal heart rate and blood pressure at peak exercise.
At each visit, maximum QRS width on 12-lead ECG and levels of NT-proBNP were recorded, when available.
Adverse cardiac outcomes during follow-up were assessed by chart review and included sustained atrial arrhythmias (defined as >30seconds of atrial flutter, intra-atrial re-entrant tachycardia, or atrial fibrillation), sustained ventricular tachyarrhythmias, and episodes of infective endocarditis (fulfilling the modified Duke criteria).13 Given the low rate of heart failure and death within the study cohort, these outcomes were not analyzed.
OutcomesThe primary outcome of the study was RVEF as measured by CMR. Secondary outcomes were: a) other parameters related to ventricular volumes and function (namely RVEDVi, LVEDVi and LVEF), levels of NT-proBNP and peak WL (in Watt) as an indicator of exercise capacity, and b) the occurrence of adverse cardiac events as defined above. Biventricular volumes and function were derived from analysis of periodic follow-up visits. Adverse events were collected in a separate file with exact dates of their occurrence.
Statistical methodsAll statistical analyses were performed using the R system for statistics and graphics, version 4.0.4.14 Descriptive statistics of the patients and adverse cardiac outcomes were tabulated by the presence and timing of PVR (no PVR, PVR before the first visit, PVR after the first visit). Longitudinal measurements of patients’ medical parameters were tabulated by the presence or absence of previous PVR (prior to a specific measurement). The data are reported medians [interquartile ranges] for continuous variables or frequencies and percentages for categorical variables. In addition, we report the proportion of missing values for each variable.
To account for missing values at individual study visits, we used multiple imputation by the R-package mice with the mice.2l.pmm imputation function from the R-package miceadds, which is based on a 2-level mixed model and predictive mean matching.15,16 We performed 100 imputations per missing value. The imputation model included several variables beyond those used in statistical analysis. Apart from age, sex, height and weight, and indicator variables for previous palliation and PVR, we also included a quadratic age term and interactions with the PVR indicator variable. Furthermore, the multiple imputation included echocardiographic measurements of LVEF, fractional area change, TAPSE and tricuspid annular systolic velocity as measured by transthoracic echocardiography, PR fraction as measured by CMR, QRS width and measures of exercise capacity (table 1 of the supplementary data). The echocardiographic measurements, LVEDVi, RVEDVi and RVEF, were omitted from the imputation because they were only performed in the University Hospital of Zurich.
The longitudinal primary outcome was RVEF measured by CMR. This outcome was analyzed using a linear mixed-effects model (R-package nlme) with patient-specific random intercepts, nested in centers.17 Age (at the corresponding visit), sex, and time-varying indicator variables for whether a patient had undergone a palliative procedure (palliation) or had undergone PVR prior to the visit, were used as explanatory variables. Each model further included an interaction term between age and PVR.
The model applied to the primary outcome was then reused to analyze the secondary functional outcomes (RVEDVi, LVEF, LVEDVi, NT-proBNP, peak WL). The outcome, NT-proBNP, is generally right-skewed and strictly positive and was log-transformed to better meet the normality assumption. Each model was fitted to each imputed dataset. The resulting coefficient estimates were then pooled according to Rubin's rules.18 To assess the impact of missing data on the results of our analyses, a sensitivity analysis was conducted for each model, using complete cases only (without imputation of missing data).
PR is the most common indication for late PVR among rTOF patients and is reliably reduced by PVR, at least in short-term follow-up after PVR. However, PR itself (determined by PR regurgitation fraction on CMR) may have an impact on the primary and secondary functional outcomes analyzed in this study (RVEF, LVEF, peak VO2, etc). To account for the effect of relevant residual PR on primary and secondary functional outcomes, we performed sensitivity analyses in which we refitted the linear mixed-effects models including PR fraction and its interaction with the time-varying PVR indicator as explanatory terms (table 2 of the supplementary data).
Adverse cardiac events (sustained atrial tachycardia, sustained ventricular tachyarrhythmias, and infective endocarditis) were analyzed using Cox proportional hazards models with the time-varying covariates, palliation and PVR, and the ordinary covariate, sex. For the time scale we used years since birth. Covariate-adjusted survival curves for patients with and without PVR were estimated from these models.
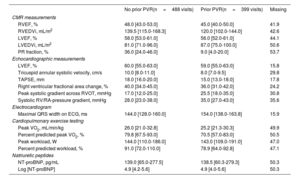
RESULTSDescriptive statisticsWe included 308 patients accounting for 887 study visits (307 patients had a follow-up visit). Table 1 presents an overview of patient characteristics stratified by PVR status (no PVR, PVR before the first visit, and PVR after the first visit). Patients who underwent PVR before their first visit were more commonly men and were younger at the first visit. Palliation prior to the intracardiac childhood repair procedure was most common among patients who underwent PVR after the first visit. The incidence of adverse cardiac outcomes stratified by PVR status is depicted in table 2. Atrial arrhythmias during follow-up were especially common in the group of patients with PVR after the first visit, followed by the group of patients with PVR before the first visit. Episodes of infective endocarditis almost exclusively affected patients with a previous PVR. Continuous ventricular function outcomes at the 887 visits, stratified by presence or absence of previous PVR, are depicted in table 3. Mean RVEF was slightly higher at visits without prior PVR and, not surprisingly, RVEDVi was also higher at visits without previous PVR. In the same line, PR fraction was considerably lower at visits after previous PVR.
Patient characteristics and follow-up stratified by PVR status
| Variable | No PVR (n=155) | PVR before 1st visit (n=105) | PVR after 1st visit (n=48) | Missing (%) |
|---|---|---|---|---|
| Male sex | 74 (48.1) | 74 (70.5) | 27 (56.3) | 0 |
| Palliation | 38 (24.5) | 35 (33.3) | 24 (50.0) | 0 |
| Age at palliation, y | 1.0 [0.3-3.1] | 0.6 [0.1-2.5] | 1.7 [0.7-3.5] | 67.5 |
| Age at intracardiac repair, y | 3.6 [1.4-6.4] | 1.9 [1.1-5.2] | 4.2 [2.1-6.7] | 0 |
| Age at PVR, y | - | 13.3 [6.1-24.9] | 32.3 [21.0-45.1] | 50.3 |
| Age at first visit, y | 29.0 [21.4-39.7] | 23.9 [19.1-32.9] | 30.5 [19.4-43.1] | 0.3 |
| Follow-up time, y | 6.3 [3.1-8.6] | 6.7 [4.5-8.6] | 9.2 [6.0-13.9] | 0 |
| Number of visits | 3 [2-3] | 3 [2-3] | 3.5 [3-4] | 0.3 |
| Any visits with CMR | 43 (27.9) | 26 (24.7) | 3 (6.3) | 0.3 |
CMR, cardiac magnetic resonance imaging; Palliation, palliative procedure prior to intracardiac repair; PVR, pulmonary valve replacement.
The data are presented as No. (%) or median [interquartile range].
Incidence of adverse cardiac outcomes stratified by PVR status
| Variable | No PVR (n=155) | PVR before 1st visit (n=105) | PVR after 1st visit (n=48) | Missing |
|---|---|---|---|---|
| IART/AFIB | 20 (13) | 20 (19) | 16 (33) | 0 |
| Atrial flutter | 13 (8) | 16 (15) | 14 (29) | 0 |
| Other SVT | 4 (3) | 5 (5) | 1 (2) | 0 |
| Ventricular tachycardia | 4 (3) | 6 (6) | 6 (13) | 0 |
| Infective endocarditis | 3 (2) | 19 (18) | 4 (8) | 0 |
| Death | 6 (4) | 3 (3) | 0 | 0 |
AFIB, atrial fibrillation; IART, intra-atrial reentrant tachycardia; PVR: pulmonary valve replacement; SVT, supraventricular tachycardia.
The data are presented as No. (%).
Descriptive statistics for measurements taken at individual visits
| No prior PVR(n=488 visits) | Prior PVR(n=399 visits) | Missing | |
|---|---|---|---|
| CMR measurements | |||
| RVEF, % | 48.0 [43.0-53.0] | 45.0 [40.0-50.0] | 41.9 |
| RVEDVi, mL/m2 | 139.5 [115.0-168.3] | 120.0 [102.0-144.0] | 42.6 |
| LVEF, % | 58.0 [53.0-61.0] | 56.0 [52.0-61.0] | 44.1 |
| LVEDVi, mL/m2 | 81.0 [71.0-96.0] | 87.0 [75.0-100.0] | 50.6 |
| PR fraction, % | 36.0 [24.0-46.0] | 9.0 [4.0-20.0] | 53.7 |
| Echocardiographic measurements | |||
| LVEF, % | 60.0 [55.0-63.0] | 59.0 [55.0-63.0] | 15.8 |
| Tricuspid annular systolic velocity, cm/s | 10.0 [8.0-11.0] | 8.0 [7.0-9.5] | 29.8 |
| TAPSE, mm | 18.0 [16.0-20.0] | 15.0 [13.0-18.0] | 17.8 |
| Right ventricular fractional area change, % | 40.0 [34.0-45.0] | 36.0 [31.0-42.0] | 24.2 |
| Peak systolic gradient across RVOT, mmHg | 17.0 [12.0-25.0] | 25.5 [18.0-35.0] | 30.8 |
| Systolic RV/RA-pressure gradient, mmHg | 28.0 [23.0-38.0] | 35.0 [27.0-43.0] | 35.6 |
| Electrocardiogram | |||
| Maximal QRS width on ECG, ms | 144.0 [128.0-160.0] | 154.0 [138.0-163.8] | 15.9 |
| Cardiopulmonary exercise testing | |||
| Peak VO2, mL/min/kg | 26.0 [21.0-32.8] | 25.2 [21.3-30.3] | 49.9 |
| Percent predicted peak VO2, % | 79.8 [67.5-93.0] | 70.5 [57.0-83.0] | 50.5 |
| Peak workload, W | 144.0 [110.0-186.0] | 143.0 [109.0-191.0] | 47.0 |
| Percent predicted workload, % | 91.0 [72.0-110.0] | 78.9 [64.0-92.8] | 47.1 |
| Natriuretic peptides | |||
| NT-proBNP, pg/mL | 139.0 [65.0-277.5] | 138.5 [60.3-279.3] | 50.3 |
| Log [NT-proBNP] | 4.9 [4.2-5.6] | 4.9 [4.0-5.6] | 50.3 |
CMR, cardiac magnetic resonance imaging; LVEDVi, left ventricular enddiastolic volume normalized to body surface area; LVEF, left ventricular ejection fraction; RVEDVi, right ventricular end-diastolic volume, normalized to body surface area; peak VO2, maximal O2-consumption on cardiopulmonary exercise testing; PR, pulmonary regurgitation; PVR, pulmonary valve replacement; RVEF, right ventricular ejection fraction; RVOT, right ventricular outflow tract; RV/RA-pressure gradient, systolic pressure gradient between right ventricle and right atrium; TAPSE, tricuspid annular plane systolic excursion.
Descriptive statistics for measurements taken at individual visits (n=887 in total), stratified by presence or absence of previous PVR. The data are presented as median [interquartile range]. Note that % means that the variable is a percentage.
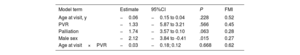
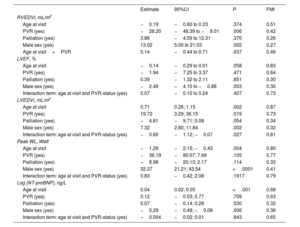
Table 4 presents coefficient estimates from a linear mixed model of RVEF. Due to multiple imputation of missing values and subsequent analysis of all completed data sets, these estimates were pooled across all analyses. Right ventricular ejection fraction was, on average, lower in patients with a prior PVR, but this association was not statistically significant (coefficient estimate: −1.33; 95% confidence interval [95%CI], −5.87-3.21; P=.566). We further assessed the interaction between age and PVR status at the visit. A significant interaction would indicate that the association of age with RVEF depends on PVR. Estimates for age and the interaction term were negative, indicating that RVEF decreased with age (estimated decrease of 0.06 percentage points per year), but at a slightly faster pace in patients with prior PVR (estimated additional decrease of 0.03 percentage points). However, neither association was statistically significant. Male sex was significantly associated with lower RVEF compared with female sex. Previous palliation was also (but not significantly) associated with lower RVEF. These findings indicate that previous PVR had no significant association on the primary functional outcome, RVEF.
Pooled coefficient estimates RVEF from linear mixed-effects model
| Model term | Estimate | 95%CI | P | FMI |
|---|---|---|---|---|
| Age at visit, y | −0.06 | −0.15 to 0.04 | .228 | 0.52 |
| PVR | −1.33 | −5.87 to 3.21 | .566 | 0.45 |
| Palliation | −1.74 | −3.57 to 0.10 | .063 | 0.28 |
| Male sex | −2.12 | −3.84 to -0.41 | .015 | 0.27 |
| Age at visit×PVR | −0.03 | −0.18; 0.12 | 0.668 | 0.62 |
95%CI, 95% confidence interval; PVR, pulmonary valve replacement; RVEF, right ventricular ejection fraction.
Linear mixed-effects model with patient specific random intercepts nested in treatment centers applied to multiply imputed data with m=100 imputations per missing value. The data contained records for 887 visits by 307 unique patients. The column FMI indicates the fraction of missing information. The intercept term for RVEF refers to the mean RVEF in % at visits in female patients at the age of the first visit, without PVR and without palliation.
Estimates from linear mixed models for the association of PVR, palliation, age and sex, and the interaction between age and PVR with the secondary functional outcomes (LVEF, RVEDVi, LVEDVi, peak WL and (log)NT-proBNP) are presented in table 5, an analog to table 4. As expected, previous PVR was associated with lower RVEDVi. Among male patients, RVEDVi was significantly higher when compared with female patients.
Pooled coefficient estimates for secondary outcome variables from linear mixed-effects models
| Estimate | 95%CI | P | FMI | |
|---|---|---|---|---|
| RVEDVi, mL/m2 | ||||
| Age at visit | −0.19 | −0.60 to 0.23 | .374 | 0.51 |
| PVR (yes) | −28.20 | −48.39 to −8.01 | .006 | 0.42 |
| Palliation (yes) | 3.86 | −4.59 to 12.31 | .370 | 0.26 |
| Male sex (yes) | 13.02 | 5.00 to 21.03 | .002 | 0.27 |
| Age at visit×PVR | 0.14 | −0.44 to 0.71 | .637 | 0.46 |
| LVEF, % | ||||
| Age at visit | −0.14 | −0.29 to 0.01 | .058 | 0.83 |
| PVR (yes) | −1.94 | −7.25 to 3.37 | .471 | 0.64 |
| Palliation (yes) | 0.39 | −1.32 to 2.11 | .651 | 0.30 |
| Male sex (yes) | −2.49 | −4.10 to −0.88 | .003 | 0.30 |
| Interaction term: age at visit and PVR-status (yes) | 0.07 | −0.10 to 0.24 | .407 | 0.73 |
| LVEDVi, mL/m2 | ||||
| Age at visit | 0.71 | 0.26; 1.15 | .002 | 0.87 |
| PVR (yes) | 19.72 | 3.29; 36.15 | .019 | 0.73 |
| Palliation (yes) | −4.81 | −9.71; 0.08 | .054 | 0.34 |
| Male sex (yes) | 7.32 | 2.80; 11.84 | .002 | 0.32 |
| Interaction term: age at visit and PVR-status (yes) | −0.60 | −1.12; −0.07 | .027 | 0.81 |
| Peak WL, Watt | ||||
| Age at visit | −1.29 | −2.15; −0.43 | .004 | 0.80 |
| PVR (yes) | −36.19 | −80.07; 7.69 | .105 | 0.77 |
| Palliation (yes) | −8.98 | −20.13; 2.17 | .114 | 0.33 |
| Male sex (yes) | 32.37 | 21.21; 43.54 | <.0001 | 0.41 |
| Interaction term: age at visit and PVR-status (yes) | 0.83 | −0.42; 2.08 | .1917 | 0.79 |
| Log (NT-proBNP), ng/L | ||||
| Age at visit | 0.04 | 0.02; 0.05 | <.001 | 0.68 |
| PVR (yes) | 0.12 | −0.53; 0.77 | .709 | 0.63 |
| Palliation (yes) | 0.07 | −0.14; 0.28 | .530 | 0.32 |
| Male sex (yes) | −0.29 | −0.49; −0.08 | .006 | 0.36 |
| Interaction term: age at visit and PVR-status (yes) | −0.004 | −0.02; 0.01 | .643 | 0.65 |
95%CI, 95% confidence interval; LVEF, left ventricular ejection fraction; LVEDVi, left ventricular end-diastolic volume normalized to body surface area; PVR, pulmonary valve replacement; RVEDVi, right ventricular end-diastolic volume, normalized to body surface area; WL, peak workload on cardio-pulmonary exercise testing.
Linear mixed-effects models with patient specific random intercepts nested in treatment centers applied to multiply imputed data with m=100 imputations per missing value. The data contained records for 887 visits by 307 unique patients. The column FMI indicates the fraction of missing information.
Furthermore, male sex was associated with lower LVEF. There was no evidence for an association between PVR and LVEF. There was an association of age with higher values of LVEDVi, but only without previous PVR, since the interaction between age and previous PVR at the visit was negative and almost canceled the association with age at visits with previous PVR. There was no significant association between peak WL and previous PVR, although the decrease in peak WL over time was slower in patients with prior PVR than in patients without PVR (not statistically significant). Finally, no association between NT-proBNP and prior PVR was found. These findings indicate that previous PVR had no significant association with the secondary functional outcomes (LVEF, LVEDVi, peak WL and NT-proBNP), except for RVEDVi.
Effect of missing information and residual pulmonary valve regurgitationBecause of a relatively high proportion of missing information in our data set, complete case analyses were performed for the primary and secondary functional outcomes as sensitivity analyses. The results of these complete case analyses were broadly concordant with the observations presented above and are shown in the supplementary data (table 3 of the supplementary data). However, since these analyses only included between 438 and 515 visits by 224 to 267 patients, the analyses using the imputed data can be considered more reliable.
Since PR may affect the functional outcomes assessed in this study, we performed another set of sensitivity analyses including PR fraction and its interaction with the time-varying PVR indicator as explanatory terms (table 3 of the supplementary data). While the main patterns remained similar as in the analyses reported in table 3 and table 4, inclusion of PR and its interaction with PVR reduced the coefficients for PVR on the outcome RVEDVi and LVEDVi and an increase in PR was itself associated with increased RVEDVi. Moreover, increased PR was associated with reduced LVEDVi, but this association was nonsignificant. The interaction between PR and PVR, which would indicate that the association of PR with the outcome depends on PVR (or vice versa), was always nonsignificant and coefficients were relatively small. This indicates that the degree of PR was not associated with primary or secondary functional outcomes (RVEF, LVEF, LVEDVi, peak WL or log NT-proBNP-levels), except for RVEDVi, irrespective of whether patients had previously undergone PVR or not.
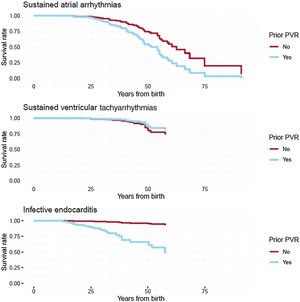
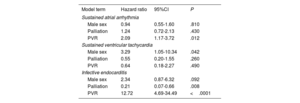
Adverse cardiac eventsHazard ratios for adverse cardiac events from Cox proportional hazards models are depicted in table 6. Covariate-adjusted survival curves by patients’ PVR status are shown in figure 1. Previous PVR was associated with an increased hazard of sustained atrial arrhythmias and even more so with infective endocarditis, but not with sustained ventricular tachyarrhythmias. The hazard for sustained ventricular tachyarrhythmias was strongly associated with male sex. Infective endocarditis was observed almost exclusively in patients with previous PVR. Interestingly, patients who had a palliation prior to intracardiac repair had a lower hazard for infective endocarditis (79% reduction in hazard).
Hazard ratio estimates from Cox proportional-hazards models for adverse cardiac events
| Model term | Hazard ratio | 95%CI | P |
|---|---|---|---|
| Sustained atrial arrhythmia | |||
| Male sex | 0.94 | 0.55-1.60 | .810 |
| Palliation | 1.24 | 0.72-2.13 | .430 |
| PVR | 2.09 | 1.17-3.72 | .012 |
| Sustained ventricular tachycardia | |||
| Male sex | 3.29 | 1.05-10.34 | .042 |
| Palliation | 0.55 | 0.20-1.55 | .260 |
| PVR | 0.64 | 0.18-2.27 | .490 |
| Infective endocarditis | |||
| Male sex | 2.34 | 0.87-6.32 | .092 |
| Palliation | 0.21 | 0.07-0.66 | .008 |
| PVR | 12.72 | 4.69-34.49 | <.0001 |
95%CI, 95% confidence interval; PVR, pulmonary valve replacement.
Survival curves for patients with and without prior pulmonary valve replacement. The curves were estimated from the Cox proportional hazards models (hazard ratio estimates in table 6) and are thus adjusted for sex and palliation. PVR, pulmonary valve replacement.
In this retrospective study of adults with rTOF, we found no evidence of a favorable association of prosthetic PVR with changes in RVEF and LVEF during follow-up. After prosthetic PVR, patients were at higher risk of sustained atrial arrhythmias and infective endocarditis during follow-up, while we found no significant difference for sustained ventricular arrhythmias (figure 2).19
Central illustration. Outcome of repaired tetralogy of Fallot: no previous PVR (n=155) vs previous PVR (n=153*). PVR, pulmonary valve replacement; VTs, sustained ventricular arrhythmias. Licensed reproduction under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License by the New Media Center of the University of Basel.19
*105 patients had PVR before the first visit, 48 patients had PVR after the first visit.
Moderate to severe PR is the most common hemodynamic residual lesion in adults with rTOF and is found in about half of all patients.1 Although the indication and timing of prosthetic PVR in patients with rTOF has been in the spotlight of congenital heart disease care for more than 2 decades, there is still no prospective randomized study demonstrating a prognostic benefit of PVR.
Recent studies in more contemporary cohorts of patients with rTOF, particularly the work provided by the group that conducts the INDICATOR-studies, have revealed important new insights into risk stratification and outcomes. Better and more detailed patient characterization with more granular data, especially including the analysis of high-quality CMR-data, failed to show an association of PR and/or right ventricular volumes with arrhythmic outcomes. Instead, right ventricular systolic dysfunction was identified as an independent predictor of adverse outcomes.5
The concept that PR leads to progressive right ventricular dilatation and dysfunction has been postulated, but is not supported by published data. Recently, a small study among adults with rTOF who had severe PR and serial CMR-studies conducted by our group suggests that for most adults with severe PR, right ventricular volumes and ejection fraction remain fairly stable during follow-up. Thus, the concept that PVR in patients with moderate to severe PR improves the long-term changes in RVEF and hence improves outcomes is currently not supported by empirical data.19
Main findings and comparison with findings of the INDICATOR cohortOur study did not demonstrate a favorable impact on long-term RVEF after PVR. This may be important, as in contrast to right ventricular volumes, RVEF has been identified as a determinant of long-term outcomes.5 While the number of deaths was small and was therefore not analyzed, we found no impact of prior PVR on the occurrence of sustained ventricular arrhythmias.
This contrasts the results of the latest report from the INDICATOR cohort. In their study, Bokma et al.7 found a significant effect of PVR on the risk of a composite endpoint, combining all-cause mortality and sustained ventricular tachycardia or resuscitated sudden cardiac death. Since their first, similar analysis published in 2018, when no significant impact of PVR on the composite endpoint had been demonstrated, in their recent analysis, a significant benefit was suggested.6,7 Compared with their initial report on 41 outcomes (4% of the study population with a mean follow-up of 5.3 years), the most recent study analyzed 82 outcomes (7% of the study population with a median follow-up of 8.0 years).6,7 Interestingly, additional events in their latest analysis were driven by an increase in all-cause mortality (30 deaths in the first analysis vs 58 deaths in the most recent report) and sustained ventricular tachycardia (6 events in the first analysis vs 18 events in the most recent analysis). Compared with their first report, only 2 additional patients died from sudden cardiac death and only 1 additional patient experienced a resuscitated sudden cardiac death.7 This is an important observation, as most deaths in the INDICATOR cohort were either from noncardiac or unknown causes (35/58, 60% of all deaths). Although the investigators of the INDICATOR cohort attempted to overcome some bias by performing a subgroup analysis, matched by a propensity score, it is important to recognize that the INDICATOR cohort is not a randomized prospective trial and the inclusion of many patients with noncardiac death may lead to substantial bias as patients with poor prognosis due to noncardiac comorbidities are unlikely to undergo PVR. Furthermore, sustained monomorphic ventricular tachycardia is often hemodynamically relatively well tolerated in patients with rTOF and may thus be a different entity from sudden cardiac death, usually caused by ventricular fibrillation or polymorphic ventricular tachycardia.20 These more organized ventricular tachycardias may be amenable to catheter ablation or arrhythmia surgery. Without careful analysis of concomitant arrhythmia surgery, which has become the standard of care at many centers, the true impact of PVR alone on the propensity of subsequent ventricular arrhythmias is difficult to estimate.
Another important difference between our study and the INDICATOR cohort is that we excluded patients with pulmonary atresia variants and other anatomies, requiring primary repair with an RV-to-pulmonary artery (RV-PA) conduit, while these patients represented 14% of the entire population in the INDICATOR cohort.5 Indeed, previous RV-PA-conduit implantation was identified as one of the strongest predictors of adverse outcomes.7 The INDICATOR-study is thus partially a study of reintervention in patients with previous PVR and outcomes may differ compared with patients with pure pulmonary valve regurgitation. The impact of the timing of PVR in patients after transannular patch repair, which inevitably leads to severe PR, typically without RVOT obstruction, has already been assessed. The authors found worse outcomes in the group of patients with early PVR than in those with late PVR. Moreover, in terms of the combined efficacy endpoint of all-cause mortality, ventricular arrhythmia, and implantation of an implantable cardioverter–defibrillator, patients with the best outcomes were those without PVR.21
Adverse cardiac eventsIn our study, prior PVR was associated with an increased risk of sustained atrial arrhythmias and an increased risk of infective endocarditis. While rates of infective endocarditis were not reported in the latest study from the INDICATOR cohort, PVR was not significantly associated with a combined secondary endpoint, consisting of heart failure, atrial arrhythmias and nonsustained ventricular tachycardias.7 The secondary endpoint, however, was driven by nonsustained ventricular tachycardias (53% of all secondary events) and a separate analysis for the individual components of the combined secondary endpoint was not provided. Interestingly, in their first report, there was a trend that early PVR (without meeting the consensus criteria) was associated with an increased risk of the secondary morbidity endpoint. Most arrhythmias in patients with rTOF are scar-related re-entrant tachycardias. Thus, differences in the propensity of atrial arrhythmias may be related to the type and size of atriotomies and the type of cannulation at the time of intracardiac repair. To reduce the long-term risk of sustained ventricular arrhythmias, direct targeting and elimination of anatomical isthmuses related to ventricular tachycardias by means of electroanatomical mapping and ablation, as proposed by the group of Katja Zeppenfeld, may be a valuable alternative to PVR for risk reduction in this feared long-term complication.21 It will certainly be of much clinical relevance to learn more from longer follow-up of the INDICATOR cohort about long-term complications. Particularly, knowing more about the impact of infective endocarditis on long-term outcomes will be of paramount importance.22
In patients with rTOF and severe PR, only a prospective randomized trial with long-term follow-up (over decades) comparing PVR vs medical therapy would be able to answer the question of whether PVR truly provides a benefit in asymptomatic patients with severe PR or whether it may even cause harm.
LimitationsThis was a retrospective observational study. Although the most frequent indication of PVR is currently RV-dilatation, due to PR (regardless of symptoms) this was not a randomized study and there may be a bias, as the indication of PVR for some of our patients may have indeed been related to symptoms. In contrast to ventricular tachycardia and endocarditis, some atrial arrhythmias may remain asymptomatic and therefore the exact date of occurrence may be slightly imprecise and hamper the precision of survival analysis. Furthermore, given the nature and analysis of our data, causality cannot be inferred from our observations and the evidence presented is purely observational. However, our findings showing no significant association of PVR with the trajectory of RVEF, as well as the increased endocarditis and atrial arrythmias among this relatively large cohort of patients with rTOF, will add to the descriptive literature on this patient population.
Although all collaborating institutions are well-established tertiary care centers for the care of adults with congenital heart disease, following national and international recommendations for care of these patients, there was no core lab for reanalysis of CMR and other clinical data.
Our models, although complex, may have inadvertently omitted variables with importance for the outcomes analyzed in our study.
The proportion of missing information in our dataset is relatively high and may affect the results. However, the fact that sensitivity analysis with complete case analyses yielded similar results indicates the relative robustness of our findings.
Our dataset does not allow determination of the exact indication for PVR in individual patients. Furthermore, for the purpose of this study, we did not analyze the timing and indication for reintervention.
In this study, we only included patients with a prior CMR study (and/or high-quality echocardiography). This might have introduced left truncation by omitting all records prior to the first CMR assessment from the analysis. Older patients and patients with contraindications for CMR (eg, electrical pacemakers or implantable defibrillators not compatible with CMR) may be underrepresented.
As this cohort was entirely obtained from a single country with a relatively homogeneous population, our results may not apply to more racially diverse populations.
Finally, SAGER guidelines on sex and gender equity in research were not taken into account during the performance of this study.23
CONCLUSIONSPrevious PVR was not significantly associated with changes in RVEF, LVEF, exercise capacity or levels of NT-proBNP in adults with rTOF. Previous PVR was associated with an increased risk of atrial arrhythmias and infective endocarditis but had no impact on the risk of sustained ventricular tachyarrhythmias. These findings should be considered when indicating PVR in asymptomatic patients with rTOF.
- –
Patients with rTOF comprise a large group of adults with congenital heart disease. A large proportion of these patients are left with severe PR.
- –
RVEF has been identified as an important determinant of adverse outcomes.
- –
It has been postulated that chronic PR leads to progressive deterioration of RVEF and that PVR may halt this process and thus improve long-term outcomes.
- –
There is, however, a lack of empirical data confirming these hypotheses.
- –
PVR was not associated with RVEF and was not associated with other functional outcomes, such as left ventricular ejection fraction, exercise capacity or levels of natriuretic peptides in a cohort of adults with rTOF.
- –
Previous PVR was, however, associated with an increased risk of atrial arrhythmias and infective endocarditis but had no impact on the risk of sustained ventricular tachyarrhythmias.
The SACHER-registry is funded by internal grants without support from the pharmaceutical industry.
ETHICAL CONSIDERATIONSThe registry and the current data analyses were approved by the local ethics committees and all patients provided written informed consent. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki. SAGER guidelines on sex and gender equity in research were not considered when conducting this study.
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCEArtificial intelligence has not been used in the preparation of this article.
AUTHORS’ CONTRIBUTIONSF.J. Ruperti-Repilado, T. Fischer, S. von Felten and M. Greutmann contributed to drafting of the manuscript, the conception of the research, critical revision of the manuscript for important intellectual content, and supervision. S. von Felten and T. Fischer where responsible for the statistical analysis. All other authors contributed to patient recruitment and data collection, and critical revision of the manuscript for important intellectual content, and supervision.
CONFLICT OF INTERESTSThere are no conflicts of interest to disclose.