Tetralogy of Fallot (TOF) is the most common cyanotic congenital heart defect. Early surgical repair has dramatically improved the outcome of this condition. However, despite the success of contemporary approaches with early complete repair, these are far from being curative and late complications are frequent. The most common complication is right ventricle outflow tract (RVOT) dysfunction, affecting most patients in the form of pulmonary regurgitation, pulmonary stenosis, or both, and can lead to development of symptoms of exercise intolerance, arrhythmias, and sudden cardiac death. Optimal timing of restoration of RVOT functionality in asymptomatic patients with RVOT dysfunction after TOF repair is still a matter of debate. Percutaneous pulmonary valve implantation, introduced almost 2 decades ago, has become a major game-changer in the treatment of RVOT dysfunction. In this article we review the pathophysiology, the current indications, and treatment options for RVOT dysfunction in patients after TOF repair with a focus on the role of percutaneous pulmonary valve implantation in the therapeutic approach to these patients.
Keywords
The overall prevalence of congenital heart disease in adults is estimated to be of 3000 per million.1 Tetralogy of Fallot (TOF) is the most common cyanotic congenital heart defect, accounting for 10% of all congenital cardiac malformations.2 Early surgical repair has dramatically improved the outcome of this condition, from a survival rate to adulthood < 25% without surgery3 to a survival of approximately 90% at 30 years in patients undergoing complete repair surgery in infancy.4 The therapeutic approaches have evolved from initial surgical palliation with Blalock-Taussig shunts5 and the first described intracardiac repair,6 an era of staged repair with shunt palliation prior to intracardiac repair, and finally an approach of direct complete repair early in infancy in the past 2 decades. Surgical techniques for complete repair evolved from a right, sometimes large ventriculotomy to close the ventricular septal defect and to resect the infundibular stenosis together with a transannular patch to relieve the right ventricular outflow tract (RVOT) obstruction to transatrial and transpulmonary approaches aiming to preserve the pulmonary valve annulus and, whenever possible, the pulmonary valve, and to minimize ventricular scarring.7,8
However, this contemporary approach with early complete repair is far from being curative and late complications after repaired TOF are frequent. In a very large cohort of patients with repaired TOF, half of the survivors had undergone a reoperation 30 years after repair.4 RVOT dysfunction is the most common complication, affecting most patients in the form of pulmonary regurgitation (PR), especially patients with transannular patches.9
In some cases, the cardiac anatomy precludes complete surgical repair, such as in patients with pulmonary atresia, absent pulmonary valve, or in the presence of an anomalous coronary artery that crosses the RVOT. In these cases, a conduit from the right ventricle (RV) to the pulmonary artery is necessary to relieve the RVOT obstruction. These conduits are also used in other types of congenital heart surgery such us in the repair of a common arterial trunk or some forms of complex transposition of the great arteries (Rastelli procedure), as well as in procedures to relieve left heart obstructions such as the Ross or Ross-Konno procedures. Degeneration of these conduits can also lead to RVOT dysfunction.
In this context, restoration of RVOT functionality often becomes necessary. Percutaneous pulmonary valve implantation (PPVI), introduced almost 2 decades ago, has become a major game-changer in the treatment of RVOT dysfunction.
In this article, we review the pathophysiology, current indications, and treatment options for RVOT dysfunction with a focus on the role of PPVI in the therapeutic approach to these patients.
PATHOPHYSIOLOGY OF RVOT DYSFUNCTIONMore than half of the patients after primary TOF repair develop RVOT dysfunction at some point in their lives. Similarly, patients with a RV to pulmonary artery conduit sooner or later experience a deterioration in conduit function leading to stenosis, regurgitation, or both.
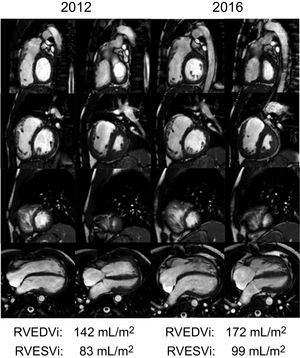
It is well known that chronic PR causes RV volume overload, which is generally well tolerated over the years,10 but if maintained over time may lead to RV dilation and dysfunction11 (Figure 1), which are in turn associated with atrial12 and ventricular arrhythmias, sudden cardiac death,13–15 exercise intolerance, heart failure, and excess mortality.16–19
Progressive dilation of the right ventricle in a patient after repaired tetralogy of Fallot and pulmonary insufficiency demonstrated by cardiac magnetic resonance imaging. Short axis stack (basal, upper row; midventricular, second row; apical, third row) and 4-chamber view (lower row) of cine steady-state free precession end-diastolic (first and third columns) and end-systolic (second and fourth columns) images of the same patient in 2012 and 2016. Note the progression of the dilation of the right ventricle. RVEDVi, right ventricle end-diastolic volume index; RVESVi, right ventricle end-systolic volume index.
In addition, residual RVOT obstruction at all levels (infundibulum, pulmonary valve, main pulmonary artery or its branches) can also contribute to RV dysfunction. Pulmonary stenosis leads to RV pressure overload and in turn to RV dysfunction due to increased RV mass:volume ratio, which has been shown to be predictive of ventricular arrhythmias and death in a large retrospective study.15
Due to this common progression to RV dysfunction of both volume- and pressure- overloaded RVs after TOF repair and its association with clinical events, the restoration of the functionality of the RVOT by means of pulmonary valve replacement (PVR) is considered when these structural changes translate into clinical problems. It is accepted that symptomatic patients with RVOT dysfunction benefit from intervention in terms of relief of symptoms,20–24 especially those patients with predominant pulmonary stenosis. However, a consistent improvement in objective functional capacity on cardiopulmonary exercise testing has not been demonstrated.20,22,24,25 Similarly, PVR improves right ventricular hemodynamic parameters such us RV size20; however, neither an increase in RV function20,22,24–28 nor an improvement in left ventricular function22,28 has been consistently demonstrated in most studies.
Although many patients may be asymptomatic for years, several studies have shown increased mortality in patients with significant PR who do not undergo surgery.29 However, neither an impact of PVR on mortality20,30 nor on late ventricular arrhythmias and sudden death has convincingly been demonstrated to date.20 Indeed, a very recent retrospective study assessing outcomes in a large cohort of patients with TOF either with or without PVR again revealed no significant differences in death and sustained ventricular tachycardia between patients with and without PVR after a mean follow-up of 5.3 years.31 In addition, late increased mortality has been reported in patients with repaired TOF even after PVR,21,30,32 probably due to the fact that RV volumes and function do not recover after PVR in a significant number patients. Therefore, a debate has emerged regarding the optimal timing for PVR, attempting to balance the benefits of an early restoration of RVOT functionality in terms of reversibility of RV structural abnormalities with the hazards of implantation of a valve with risk of degeneration and the need for multiple subsequent surgeries for valve replacement in the future.
CURRENT INDICATIONS AND TIMING FOR RESTORATION OF RVOT FUNCTIONALITYDue to the deleterious effects of RVOT dysfunction on RV function, current therapeutic approaches aim to avoid a too late operation by intervening when the RV structural abnormalities are still reversible.33
Current European guidelines recommend PVR for symptomatic patients with RVOT dysfunction after TOF repair in the form of severe PR and/or severe pulmonary stenosis (defined as a RV systolic pressure > 60mmHg). Quantification of PR can be challenging and is beyond the scope of this review.34 Similarly, addressing clinical symptoms is sometimes not straightforward in this group of patients and cardiopulmonary exercise testing plays a major role in the evaluation of the symptom status and cardiopulmonary reserve in this context.35
The indications for restoration of RVOT functionality in asymptomatic patients with RVOT dysfunction after TOF repair remain a major controversial issue in congenital cardiology. Current European guidelines recommend intervention in the presence of severe PR or pulmonary stenosis and a decrease in objective exercise capacity in cardiopulmonary exercise testing, progressive RV dilation, progressive decline in RV systolic function, progressive (at least moderate) tricuspid regurgitation, very severe RVOT obstruction with RV systolic pressure > 80mmHg, and sustained atrial or ventricular arrhythmias.36
Because indications for surgery in asymptomatic patients are largely based on RV structural abnormalities, their evaluation plays a paramount role in selecting candidates who may benefit from surgery. Although echocardiography remains an important first-line modality for this purpose, RV geometry and its retrosternal position preclude an accurate assessment with this technique alone. Due to its reproducibility and excellent spatial resolution, cardiac magnetic resonance imaging has emerged as the cornerstone of the evaluation of RV structural abnormalities after TOF repair.34
However, the timing of the intervention in asymptomatic patients with known RV structural abnormalities remains challenging. The time course of RV dilation and dysfunction in patients with RVOT dysfunction is still not well understood. In this context, current guidelines recommend a close follow-up in specialized centres to detect progression of structural abnormalities in asymptomatic patients.36 It has been shown that RV volumes and function remain stable in most patients.37,38 A recent study showed that RV dilatation and dysfunction as well as left ventricular dysfunction progress slowly in most patients after TOF repair. However, in approximately 15% of the patients, substantial worsening occurred in ventricular parameters and was not easily predictable.38 A watchful-waiting strategy has traditionally been adopted in most asymptomatic patients because of the risk of multiple major cardiac surgeries, which has been deemed to be too high in this population. In addition, a more aggressive approach to restore RVOT functionality in asymptomatic patients based on cardiac magnetic resonance imaging-derived RV volumetric data has to date not demostrated to improve outcomes.
A number of studies have attempted to elucidate the optimal cutoff for RV dilation indicating intervention and to determine the best parameter to monitor RV performance over time. A study by Geva et al.24 showed that a reduced RV ejection fraction < 45% was associated with persistent RV dysfunction after PVR. However, ejection fraction can be preserved in volume-overloaded ventricles in which pathologic remodelling is already present. Cardiac magnetic resonance imaging-derived RV end-diastolic and end-systolic volumes have been extensively studied as indicators of pathologic remodelling and many efforts have been made to find a critical threshold of indexed RV end-diastolic (RVEDVi) and end-systolic (RVESVi) volume above which complete reverse remodelling is no longer achievable and therefore under which intervention should be indicated. These proposed cutoff points were progressively reduced from EVEDVi > 170mL/m2 or RVESVi > 85mL/m2 in the study by Therrien et al.39 to RVEDVi > 160mL/m2 by Oosterhof et al.26 Lee et al.28 proposed cutoffs of RVEDVi < 168mL/m2 and RVESVi < 80mL/m2. In the past few years, greater focus has been placed on RV end-systolic volume, establishing it as a more important indicator of RV hemodynamic performance. A recent study by Bokma et al.40 showed that undergoing PVR with a preoperative RVESVi under 80mL/m2 was associated with normalization of RV volumes and that a too late intervention with RVESVi > 95mL/m2 was associated with adverse clinical events. More recently, Ling Hen et al.41 showed that significant reverse remodelling takes place immediately after PVR with reductions in both RVEDVi and RVESVi, followed by a continuing process of further biological remodelling reflected by further reduction in RVESVi, underscoring the role of this parameter to monitor myocardial function in this context, proposing a RVESVi < 82mL/m2 as the best cutoff for normalization of RV function, in accordance with previous reports.
Some groups have proposed even lower thresholds for intervention. A study by Frigiola et al.22 showed a higher rate of normalization of RV volumes and an improvement in biventricular function accompanied by an increase in exercise capacity using a more liberal approach, with surgery being performed when RVEDVi exceeded 150mL/m2. However, this remains controversial, as a more liberal approach in asymptomatic patients may also have unwanted consequences. Bokma et al.31 showed that patients undergoing PVR at a lower volumetric threshold (RVEDVi under 160mL/m2) had a higher event rate of heart failure, atrial arrhythmia, and nonsustained ventricular tachycardia.
Another aspect that may influence the timing of intervention in these patients is the hypothesis supporting that the effects of restoration of RVOT functionality may be influenced by patient age at intervention. In the study by Frigiola et al.,22 objective improvements in functional capacity were more likely to be achieved in patients who underwent surgery when younger than 17 years. This more liberal approach regarding age has also been questioned. A recent study showed that PVR before the age of 16 years did not improve event-free survival compared with PVR after 16 years of age.42 Complications, including mortality, endocarditis and re-do PVR, occurred significantly earlier in patients with PVR before 16 years of age.
CURRENT THERAPEUTIC APPROACHES TO RESTORE RVOT FUNCTIONALITYIn most patients after primary TOF repair, surgical PVR is the treatment of choice, as it has been shown to improve pulmonary blood flow, reduce tricuspid regurgitation, and improve RV mechanics, resulting in clinical improvement.18,22,24,31 Pulmonary valve replacement can be performed with low early and late mortality in both the pediatric and the adult population.43 Recent series report a perioperative mortality as low as 1% in the current era.43–45 Several surgical options to restore RVOT functionality are available.
Mechanical valves in the pulmonary position are associated with complications mostly related to the need for chronic anticoagulation and the potential for valve thrombosis. They are rarely implanted to restore RVOT functionality, despite higher durability.46 In addition, they preclude further access to the pulmonary circulation in case interventions in the pulmonary vasculature become necessary.
Among the tissue valve options available for PVR, valved homografts, valved bovine jugular vein conduits, and stented or stentless bioprosthetic porcine and bovine pericardial valves are the preferred options due to their lower risk of thrombosis and lack of need for systemic anticoagulation. However, patients requiring this type of valves, either as part of the primary repair or as a secondary intervention to treat RVOT dysfunction are at risk of valve failure due to degeneration.
Aortic and pulmonary homografts were historically the most commonly used valves. A major drawback is their limited availability and high cost, as well as their accelerated degeneration, especially in younger patients, who may have an enhanced immune response.47,48
A relatively common alternative to homograft conduits for RVOT reconstruction are bovine jugular vein conduits. However, similarly to homografts, these conduits have limited durability. Although 1 study found bovine jugular vein conduits to have superior durability to homograft conduits, most studies have shown no significant differences in performance between the conduit types.49–52
Finally, bioprosthetic valves are available in a wide range of sizes and are the preferred option for adults undergoing surgical PVR. It is well known that bioprosthetic valves in the pulmonary position degenerate and lead to failure.21,27,47,53 This limited durability is related to valve type and age at implantation, with a median durability of approximately 15 years if implanted in the third decade of life.53,54
The perfect surgical pulmonary valve implant does not exist and virtually all patients receiving tissue valves or conduits for primary repair or secondary PVR will face several reinterventions due to degeneration.
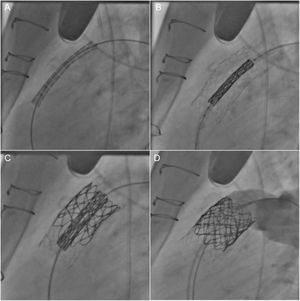
With the advent of PPVI in 2000,55 the therapeutic approach to restoration of RVOT functionality has undergone a significant change. This technique has the advantage of avoiding resternotomy and cardiopulmonary bypass and has become an attractive alternative to surgical PVR in selected patients (Figure 2).
For appropriately selected candidates, mainly those with a previously implanted RV-pulmonary artery conduit, PPVI has been shown to be a safe and reliable option for restoration of RVOT functionality, with a low incidence of post-procedural PR, a reduction in patient symptoms, an improvement in right ventricular hemodynamic parameters and an improvement in functional capacity.56–59 Currently there are 2 devices widely used for PPVI.
The Melody valve (Medtronic Inc), consists of a platinum stent in which a bovine internal jugular vein valve is inserted. Two diameters are currently available, 20mm and 22mm, which can be implanted in conduits ranging from 16mm to 22mm.
The Edwards Sapien valve (Edwards Lifesciences Corp, Irvine, CA) is a bovine pericardial valve within a balloon-expandable stent. The system was originally developed for transcatheter aortic valve implantation and was first used in the pulmonary position in 2006.60 Current developments of the system, with second- and third-generation prostheses (Sapien XT and Sapien 3, respectively) are already available. Both use cobalt chromium stents and are being used for PPVI with a range of valve sizes from 20mm to 29mm.61
In recent years, the experience has become broader and the results are promising. PPVI with the Melody valve provided good hemodynamic and clinical outcomes up to 7 years after implantation, with 5-year freedom from reintervention and explantation of 76% and 92%, respectively.62 Although usually implanted through the femoral vein, alternative access routes such as jugular or transhepatic can be successfully used in patients with venous obstructions.63 However, this technique is not free of complications and a number of technical factors play a major role in clinical outcomes. In earlier experiences, stent fracture due to the anterior position of the valve in the thorax with increased mechanical stress was a common cause of valve failure. The current almost universal use of prestenting has dramatically decreased this complication.62 Small patients with small conduits are a challenging group, and despite the good results of PPVI in the pediatric population,64 conduit rupture or perforation can occur. This complication can be overcome with the bail-out use of covered stents. Coronary obstruction due to compression at the time of implantation can occur in up to 5% of patients.65 A careful evaluation of the coronary anatomy with simultaneous balloon inflation is necessary to avoid this complication. Endocarditis can be a major complication with rates reported up to 2.4%.66 Patients treated with percutaneous pulmonary valves are exposed to other, less frequent complications currently better known and studied in patients treated with transcatheter valves in aortic position. Noninfective valve thrombosis has been reported after PPVI and, although commonly resolved with anticoagulation, it can represent significant morbidity in this population.67–69
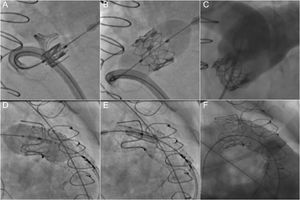
The need for a stable landing zone to anchor the valve has limited PPVI to approximately 15% of patients with RVOT dysfunction.70 However, this approach has become an attractive option for secondary RVOT interventions in patients with a bioprosthetic valve, as valve-in-valve implantations can avoid reoperations in these patients71,72 (Figure 3). New developments may allow an expansion of the indications to patients with native RVOTs with larger diameters and without previously implanted conduits or valves.73–75 In addition, the use of modern percutaneous valves with diameters up to 29mm allow the treatment of larger dysfunctional RVOTs.61 Moreover, the more widespread use of modern surgical approaches respecting the pulmonary valve at the expense some of residual stenosis can increase the number of potential candidates for this technique.
Percutaneous pulmonary valve implantation in a dysfunctional bioprosthesis (valve-in-valve). A-C: implantation of a Sapien XT valve in a Perimount bioprothesis without prestenting. D-F: implantation of a Sapien 3 valve in a Carpentier-Edwards bioprosthesis with prestenting to prepare the landing zone.
Nevertheless, there is still a large number of patients with dilated RVOTs due to extensive transannular patches in which the placement of a percutaneous device remains a challenge and they are usually referred to surgical PVR. Strong research efforts are focusing on new devices to expand percutaneous techniques to these patients. Promising results have been shown by the Venus P valve (Med Tech, Shanghai, China), consisting of a self-expanding nitinol stent with a porcine pericardial valve with proximal and distal expansions, and the Harmony transcatheter pulmonary valve (Medtronic Inc, Minneapolis, MN), which is a 22mm porcine pericardial valve sewn to an asymmetric self-expanding stent of nitinol, also with larger proximal and distal ends to accommodate different RVOT morphologies.76–78
RETHINKING THE TIMING OF RVOT REINTERVENTION IN THE ERA OF PPVICurrent European guidelines recommend PPVI with the same indications as PVR in suitable candidates.36 In practice, PPVI is usually offered as the first-line option to patients with RVOT dysfunction who are technically suitable candidates, as these patients are generally poor surgical candidates. However, no randomized clinical trial has compared surgical PVR to transcatheter PPVI head-to-head and it still remains unclear whether PPVI should be offered over surgical PVR in patients who are eligible for surgery and are at low operative risk. In addition, only patients with conduits or previous PVR are usually technically suitable for PPVI, while most of those with native RVOT are currently not. Despite current applicability to only a subgroup of patients with RVOT dysfunction, the advent of PPVI has led to a paradigm change in the general approach to restoration of RVOT functionality after TOF repair beyond the “competition” of both techniques.
As previously mentioned, one of the major pitfalls of TOF repair is the need for restoration of RVOT functionality at some point after repair. Most patients will receive a valve or conduit which will inevitably degenerate over the years, leading to several reoperations over a lifetime, with associated morbidity and mortality.14,66
This fact has contributed to a certain resistance among congenital cardiologists to refer patients with RVOT dysfunction for surgical PVR as long as they are asymptomatic. The possibility of performing PPVI as a valve-in-valve procedure in a degenerated bioprosthesis can avoid reoperations and has become largely accepted in this clinical scenario,71,72 as PPVI can be performed with a very low risk in the current era. The most contemporary series, accounting for the current almost universal practice of extensive prestenting, shows excellent short- and mid-term outcomes.79 In this regard, a recent meta-analysis of 19 studies including 1044 patients undergoing transcatheter pulmonary valve implantation reported a procedural success rate over 96% with a conduit rupture rate of 4.1% and coronary complication rate of 1.3%. The incidence of reintervention was 4.4 per 100 person-years overall and was significantly lower in studies reporting higher rates of prestenting.80 In addition, patients with a stenotic transcatheter pulmonary valve (for example due to stent fracture) could also benefit from a percutaneous reintervention.62
However, the number of percutaneous re-do procedures is limited as the effective maximum internal diameter of the conduit or bioprosthesis is inevitably decreased after each valve placement. Additionally, although long-term data are lacking, percutaneous pulmonary valves are expected to degenerate after several years similarly to their surgical counterparts. In addition, it remains to be demonstrated whether percutaneous valves impact survival by avoiding new operations. Moreover, it seems unlikely that percutaneous valves avoid surgery at all in these patients. Increased age, and eventually increased comorbidities may confer a higher risk for the probably unavoidable intervention. In addition, the presence of numerous stents and valves in the RVOT may increase surgical complexity and therefore surgical risk.
Nevertheless, the availability of a percutaneous alternative to surgery with a low risk profile for degenerated valves seems to support earlier intervention in patients with RVOT dysfunction after TOF repair, even if surgery is the first-line option before RV structural abnormalities become irreversible. If symptoms or RV structural abnormalities fulfilling the criteria for PVR occur during late childhood or adolescence often an adult-sized bioprosthesis can be inserted. At this point surgeons should take into account the possibility of implanting a subsequent percutaneous valve in case there is degeneration of the surgical valve when choosing the valve type (stented porcine or bovine pericardial bioprosthetic valve instead of cryopreserved homografts) and the valve size (at least 25mm), to allow the implantation of subsequent percutaneous valves. If this is not possible because the patient's chest cannot accommodate such a valve, a bovine jugular vein conduit can be used, as recent data show that PPVI is feasible even in the lowest spectrum of sizes.81
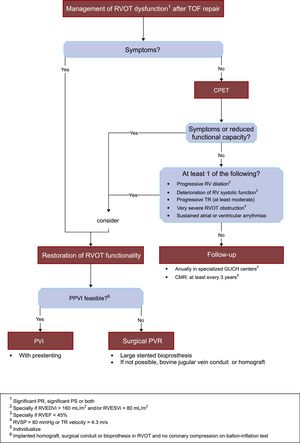
Figure 4 summarizes in an algorithm our approach to the management of RVOT dysfunction in patients after TOF repair.
Proposed algorithm for the management of RVOT dysfunction after TOF repair. CMR, cardiac magnetic resonance imaging; CPET, cardiopulmonary exercise test; GUCH, grown-up congenital heart disease; PPVI, percutaneous pulmonary valve implantation; PR, pulmonary regurgitation; PS, pulmonary stenosis; PVR, pulmonary valve replacement; RV, right ventricle; RVEDVi, right ventricle end-diastolic volume index; RVEF, right ventricular ejection fraction; RVESVi, right ventricle end-systolic volume index; RVOT, right ventricle outflow tract; RVSP, right ventricle systolic pressure; TOF, tetrallogy of Fallot; TR, tricuspid regurgitation.
The timing of restoration of RVOT functionality in patients with RVOT dysfunction after TOF repair remains controversial. However, the advent of PPVI has provoked a paradigm shift toward an earlier RVOT repair for PR, even if surgery is required in a first step. Once an appropriately sized bioprosthetic ring has been implanted, PPVI is feasible, with low risk and good short- to mid-term outcomes. Whether an earlier PVR and subsequent PPVI to avoid reoperations indeed impacts outcomes has, however, yet to be demonstrated.
CONFLICTS OF INTERESTF. de Torres-Alba declares having received congress travel support from Edwards Lifesciences. G. Kaleschke declares having received congress travel support from Edwards Lifesciences and Medtronic. H. Baumgartner declares having received congress travel support from Edwards Lifesciences, Abbott and Medtronic, and speaker fees from Edwards Lifesciences.
.