Keywords
INTRODUCTION
The risk of prosthetic valve endocarditis is not uniform--it is higher during the first 6 months after valve surgery (particularly in the first month and a half).1 In contrast to native valve endocarditis, prosthetic valve endocarditis tends to extend beyond the valvular ring, up to the annular and periannular tissue, as well as the mitral-aortic intravalvular fibrous cap, causing periannular and septal abscesses, fistulas, and prosthetic damage with periprosthetic regurgitation that is hemodynamically relevant. The major invasive risk of endocarditis tends to be in cases of aortic localization with an early clinical course.2,3 Infectious prosthetic valve endocarditis has a high mortality rate (greater than 30%) that increases to up to 55% when accompanied by an aortocardiac fistula.3
Aortoccardiac fistulas are present in approximately 2% of patients with infectious endocarditis, as has been described in a 10-year consecutive series of 346 patients.4 Echocardiography, especially transesophageal echocardiography, tends to be the diagnostic procedure of choice for these endocarditis complications, despite the fact that other imaging techniques are used for diagnosis.5,6
We present a patient with early prosthetic endocarditis secondary to Staphylococcus epidermidis with very aggressive progression, that was refractory to medical treatment and ended in death, despite attempted surgical intervention.
CLINICAL CASE
A 53-year-old man, an ex-smoker, was operated on 2 months previously for implantation of a replacement Omnicarbon 23 aortic valve prosthesis because of aortic stenosis in the bicuspid valve and aortocardiac right saphenous bypass for single-vessel coronary disease in the middle third of the right coronary artery.
The patient was admitted to the emergency room of another medical center secondary to a 15-day history of dyspnea, general malaise, and chills, with a fever of 38.5ºC upon arrival. Physical examination including cardiac auscultation revealed a 4/6 diastolic murmur and pulmonary auscultation revealed bilateral crackles up to middle field. Also noted was bilateral malleolar edema.
Blood work revealed 32 700 leukocytes/µL, with 92% neutrophils; hemoglobin 9.1 g/dL; and 87 000 plaques/µL; and biochemistry showed a creatinine of 1.1 mg/dL; urea 675 mg/dL; glucose 123 mg/dL; sodium 128 mEq/dL; and potassium 3.3 mEq/dL. Chest radiography revealed bilateral alveolinterstitial infiltrates.
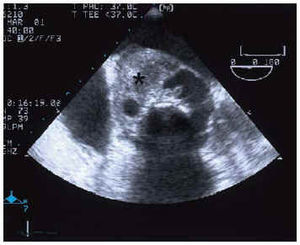
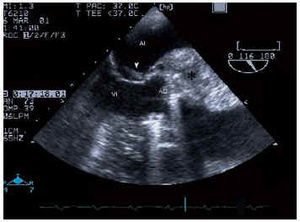
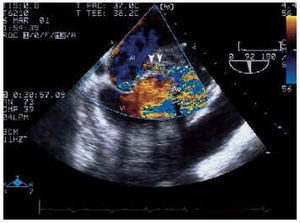
Electrocardiogram showed a sinus rhythm, right branch blockage, long PR and signs of left ventricular hypertrophy. Suspecting endocarditis, blood culture and a transthoracic echocardiogram were performed, which revealed good LF function with concentric hypertrophy, slight dilatation, prolific aortic microorganism growth, with a perivalvular abscess and a severe aortic insufficiency signal (TAP 100 ms and jet width 15 mm) with prosthetic damage. A transesophageal electrocardiogram was subsequently performed which confirmed the previous findings and also identified the abscess (Figure 1) with fistulization between the aorta and left atrium and with adjacent microorganism growth on the anterior valve of the atrial face of the mitral valve (Figures 2 and 3).
Fig. 1. Transesophageal echocardiogram at 0º, in the aortic root, with a large abscess (*) occupying half the diameter of the root. Ao indicates aorta.
Fig. 2. Transesophageal echocardiogram at 116º showing the periannular abscess (*) and microorganism growth located in the anterior mitral valve (arrow). LA indicates left atrium; LV, left ventricle.
Fig. 3. Transesophageal echocardiogram in the same plane as Figure 2 where, with color Doppler, the fistula that connects the coronary sinus with the left atrium can be observed (arrow). LA indicates left atrium; LV, left ventricle.
Treatment7 was initiated with vancomycin, gentamycin, and rifampicin, and S epidermis was grown from blood cultures. The patient was admitted to the intensive care unit, transferred to the cardiac surgery service of another hospital for treatment with aortic homograph, and died during the procedure. During surgery a large (6-8 mm diameter) aortic valve abscess full of infectious thrombotic material was found, with destruction of the left atrium roof, interventricular membranous subaorta septum, prosthetic damage and fragility of the native aorta wall. Occlusion of the right coronary opening by the permeable saphenous graft was also observed.
DISCUSSION
This case illustrates the aggression with which certain microorganisms can affect patients with prosthetic valves. This is especially prevalent in the case of early endocarditis (less than 60 days after replacement surgery) and in those cases involving the valve implant, the mechanical prostheses are those that are most likely to become infected; after 12 months, the risk of infection of a bioprostheses is greater.1 There are references in the literature to complications of prosthetic endocarditis, and, in particular, those resulting from abscesses and fistulas in surrounding cavities.4,9,10 In this case, a series of observations were made that have not been previously described in a single patient, such as a large proliferant value abscess mass, severe aortic insufficiency, a fistula toward the left atrium with protrusion of microorganism growth into the anterior mitral valve cavity, rupture of the left atrium roof, and an interventricular membranous septum (the last 2 were found during surgery). Prosthetic valve endocarditis diagnosed 60 days after surgical implant tends to be caused by Staphylococcus coagulasa negative, above all S epidermidis. Other less common organisms include staphylococcus aureus, gramnegative bacillus, and the fungi.
Echocardiography is the diagnostic method of choice for valve disease and, in the case of inflammatory disease with the presence of microorganisms, it is especially important because of the potential for visualization of morphologic changes and eventual microorganism growth on the valve, and also because it allows detection of other changes. Color Doppler images best show the presence of fistulas. The transesophageal technique provides the best diagnostic information,11 especially for prosthetic valve endocarditis. In our case it allowed us to observe the large amount of inflamed matter on the aortic prosthesis, which extended toward the whole ring, encrusting it through a fistula towards the left atrium, creating doubt initially as to whether the anterior mitral valve was affected by the infectious process (A more detailed visualization allowed us to see that the valve was affected and permitted us to assess the severity of the aortic insufficiency). During surgery we observed the rupture of the left atrium roof and an interventricular membranous septum, both of which developed after the echocardiogram (10 days had elapsed between the 2 studies because the patient had been referred to another center for a homograft procedure). The role of surgery in the treatment of prosthetic endocarditis, is that it must be performed early, taking into account the clinical and hemodynamic status of the patient. The presence of cardiac failure secondary to valvular regurgitation, signs of myocardial or periannular invasion, dysfunction of the prosthetic valve, sepsis refractory to appropriate antibiotic treatment, repeated embolism, or mycotic endocarditis by S aureus, are all indications for early intervention. It is clear that full spectrum antibiotic therapy should initially be administered to all patients with endocarditis, and progression of the illness or signs of cardiac failure should be the criteria for surgical intervention.12 Determining the type of surgery to be performed is the subject of controversy, as choosing the convenience of using a homograft over the implantation of a mechanical or even biological prosthesis is debatable.12,13 In studies that established the graft as the preferred technique for surgical treatment of endocarditis, initial results appear to be promising, although the number of patients followed is not very large. The use of grafts is left for serious cases and requires a high level of expertise on the part of the surgical team involved. Surgical mortality and the incidence of reinfection appear to be reduced with the graft. Moon et al3 describe the promising results obtained with bioprostheses in patients more than 60 years of age with native as well as prosthetic endocarditis. This procedure is also of use in younger patients with limited associated disease. In any case, it seems that the most currently accepted method is the implantation of a mechanical prosthesis in patients who present with endocarditis.14 We await more conclusive results and further studies on the use of grafts.
Correspondencia: Dr. J.R. Ortega.
Hospital de Gran Canaria Dr. Negrín.
Bco. La Ballena, s/n. 35020 Las Palmas de Gran Canaria. Spain
E-mailo: jortega@correo.hpino.rcanaria.es