Keywords
INTRODUCTION
Hypertrophic cardiomyopathy (HCM) is the most common form of hereditary cardiomyopathy. Characterization of the disease by molecular analysis has identified mutations in genes coding for the sarcomere proteins, and histological study has shown anomalous arrangement of the myocardial fibers, ventricular hypertrophy, myocardial vessel abnormalities, and fibrosis.1 As to the clinical manifestations, in addition to intercurrent complications such as stroke,2 the severity of the disease is dictated by 2 features: first, hemodynamic deterioration and limitations in exercise capacity due to diastolic dysfunction, and, on occasion, altered systolic function with clinical heart failure; and second, the risk of ventricular arrhythmia and sudden death.3-5 Nonetheless, our knowledge of the predictors of hemodynamic deterioration and sudden death in this disease is limited. The degree of functional deterioration can be weakly predicted by the magnitude of hypertrophy, ventricular obstruction, and other classic markers of disease severity.6 Concentrations of N-terminal pro-B-type natriuretic peptide (NT-proBNP) show a better (inverse) correlation with functional class7 and maximum oxygen consumption during exercise.8
Cardiac magnetic resonance (CMR) imaging has been recently applied to the study of HCM.9-14 In addition to a detailed morphologic and functional examination, intravenous administration of gadolinium allows detection of areas showing late gadolinium enhancement (LGE). Gadolinium remains in the extracellular matrix 10 to 20 minutes after it is injected, accumulating in areas of fibrosis5 and (possibly to a lesser degree) in areas with considerable structural loss.13,16 Thus, fibrosis can be investigated in vivo. Up to now, the contribution of myocardial fibrosis to the functional limitations of HCM patients has not been established.
The aim of this study was to determine whether LGE on CMR imaging is independently associated with the limited functional capacity of patients with HCM. To this purpose, we included the most important markers related to functional capacity, such as clinical and echocardiographic parameters and baseline NT-proBNP concentrations.
METHODS
Patients
The study included 98 consecutive patients diagnosed with HCM at the dedicated clinics of Hospital Universitario Virgen de la Arrixaca (Murcia, Spain) (44 patients) and Hospital General Universitario de Alicante (Alicante, Spain) (54 patients). Patients who had a contraindication, or were unable to undergo exercise testing or CMR imaging, were excluded.
The inclusion criteria required a diagnosis of HCM according to current guidelines4: left ventricular wall thickness ≥15 mm in the absence of another cause of ventricular hypertrophy; in the case of affected first-degree relatives, current proposed criteria were followed.17
All patients underwent clinical assessment, contrast-enhanced CMR imaging, conventional treadmill exercise testing, and 24-hour Holter monitoring to evaluate possible nonsustained ventricular tachycardia (NSVT). In a subgroup of 71 patients, baseline NT-proBNP concentrations were additionally obtained.
Clinical Assessment
A complete clinical evaluation was performed in all patients. Special emphasis was placed on the presence of dyspnea (NYHA class), angina, syncope, and atrial fibrillation, and other rhythm alterations. In addition, a risk stratification study for sudden death was carried out, assessing the following factors: a) family history of sudden death; b) recurrent syncope of unexplained origin or resuscitated sudden death; c) abnormal blood pressure response on exercise testing; and d) NSVT on Holter monitoring and maximum left ventricular thickness ≥30 mm on echocardiography, or a resting left ventricular outflow tract (LVOT) gradient >30 mm Hg.
Doppler Echocardiography
Echocardiography was carried out with a Sonos 5500 unit (Phillips, Eindhoven, The Netherlands). The examinations were performed with the patient at rest and the images were stored in digital format for later review. Wall thickness was measured in short-axis views at the level of the mitral valve and papillary muscles, seeking the greatest thickness of the left ventricular wall. The ejection fraction was calculated with the Simpson method by averaging the values from 2- and 4-chamber views. Diastolic function was determined by pulse Doppler of the mitral valve inflow, as well as by the peak diastolic velocities of the mitral annulus and pulmonary venous flow. Presence of an LVOT gradient was determined by color Doppler and continuous wave Doppler; values >30 mm Hg were considered significant.
Exercise Testing
Exercise testing was performed with a treadmill (Marquette Electronics Inc., Milwaukee, USA). Symptom-limited exercise testing was performed in all patients (88 with the Bruce protocol and the remaining 10, who had difficulty walking, with the modified Bruce protocol). Blood pressure was determined noninvasively while the patient was at rest, and every minute during exercise testing and during the 5 minutes thereafter. Blood pressure response was defined as abnormal when it did not exceed the resting pressure by at least 20 mm Hg or when there was a more than 20-mm Hg decrease relative to the peak recorded during exercise testing.18
Oxygen consumption was indirectly estimated in metabolic equivalent units (METs) according to the standard formulas included in the equipment software.
Cardiac Magnetic Resonance Imaging
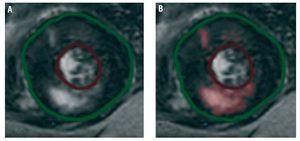
Cardiac magnetic resonance imaging was carried out with a 1.5-T scanner (Signa LX, General Electric), with a 6-element torso phased array coil and cardiology software (version 9.1). Gadolinium was administered in a 0.2-mm/kg bolus dose. Ten minutes later, gradient-echo sequences were acquired, with inversion recovery in multiple slices to assess the presence of myocardial fibrosis. The resulting images were processed with the Mass Suite program (version 6.1) from Medis (Leiden, The Netherlands). The presence or absence of fibrosis was described. In addition, the percentage of myocardium presenting late gadolinium uptake was measured by digital quantification. For this purpose, areas with positive uptake were defined as signal intensity 2 SD above the mean signal in a distant sample of healthy myocardial tissue (Figure 1).
Figure 1. Process used to quantify affected tissue by late gadolinium enhancement. A: before processing. B: areas presenting signal intensity 2 SD above values recorded in a sample of healthy myocardial tissue are automatically selected (shown in pink).
NT-proBNP Determination
Blood samples were drawn from the antecubital vein with the patient at rest and following a 12-hour fast. Samples were centrifuged at 3500 g for 15 minutes and the resulting sera were stored at -40°C until analysis. NT-proBNP concentration was determined with a diagnostic kit from Roche on an Elecsys 2010 analyzer (Roche Diagnostics, Mannheim, Germany). The lower and upper detection limits were 0.6 pmol/L and 4130 pmol/L, respectively. To convert pmol/L to pg/mL, pmol/L values were multiplied by 8457.
Statistical Analysis
The Kolmogorov-Smirnov test was used to determine whether the variables analyzed followed a normal distribution. Variables having a normal distribution were expressed as the mean (SD), and those having a non-normal distribution as the median and interquartile range. Qualitative variables are expressed as percentages.
Relationships between the exercise capacity (MET) and the different variables were investigated. The Student t test was used for dichotomous variables and correlation analysis for continuous variables (Pearson coefficient for those with a normal distribution and Spearman coefficient for those with a non-normal distribution). For the analysis of NT-proBNP concentrations, which did not follow a normal distribution, logarithmic transformation was carried out.
Lastly, to study potential confounding factors and the importance of the independent variables over the others, the most significant variables (P<.150 in the univariate analysis) were entered in a multivariate linear regression analysis model. A P value less than .05 was considered significant. SPSS (version 11, SPSS Inc.) was used for the statistical analyses.
RESULTS
A total of 98 patients (age, 46.3 [15.4] years; 71.4% men) were included in the study. Baseline characteristics of the study population are shown in Table 1. Among the total, 61% of patients presented dyspnea (NYHA functional class II or greater). The mean value for exercise capacity recorded on exercise testing was 8.8 (3.7) MET. In addition, 52% of patients had 2 or more factors for sudden death and 68.4% showed LGE on CMR imaging.
In the studies to determine the relationships between the clinical variables and MET findings on exercise testing (Table 2), functional capacity was found to be poorer in women, patients with dyspnea, and those with a history of syncope, hypertension, or atrial fibrillation. The additional tests revealed poorer functional capacity in patients with ventricular obstruction (>30 mm Hg), abnormal blood pressure response on exercise testing, and LGE on CMR imaging. There was no relationship between exercise capacity and maximum left ventricular wall thickness ≥30 mm (although only 5 patients presented values above this cut-off), NSVT, or a family history of sudden death.
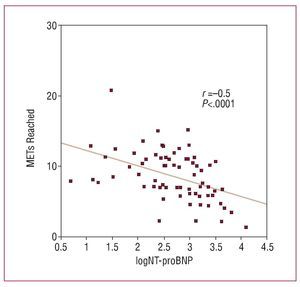
Correlations between the quantitative variables and MET findings (Table 3) showed a significant inverse relationship between exercise capacity and age, plasma NT-proBNP value, degree of LVOT obstruction, maximum left ventricular wall thickness, anteroposterior diameter of the left atrium, and percentage of affected myocardium, as determined by LGE. The left ventricular end-systolic diameter was directly related to functional capacity. No statistically significant correlation was found between the left ventricular ejection fraction and the MET values attained. The logarithm of NT-proBNP (logNT-proBNP) was the variable that correlated best with functional capacity (r=-0.5; P<.0001) (Table 3, Figure 2).
Figure 2. Correlation between exercise capacity values (METs) attained on exercise testing and the logarithm of plasma NT-proBNP concentrations. Following logarithmic conversion, logNT-proBNP presented a normal distribution. The significance level and r value by Pearson correlation analysis are indicated.
Variables Related With Late Gadolinium Enhancement
Patients showing LGE on CMR imaging presented severe dyspnea (NYHA III-IV), and NSVT on Holter monitoring more often, and showed higher concentrations of NT-proBNP. On echocardiography, patients with LGE presented greater wall thickness, more severe ventricular obstruction, and smaller left ventricular end-systolic and end-diastolic diameters. Nonetheless, in our sample there was no relationship between LGE and the left ventricular ejection fraction (data not shown).
Late Gadolinium Enhancement and Functional Capacity
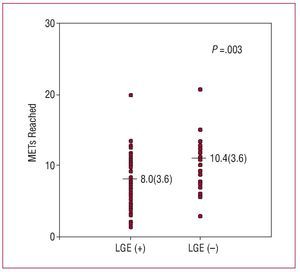
Late gadolinium enhancement on CMR imaging was related to poorer exercise capacity (8.04 [3.56] vs 10.41 [3.57] MET; P=.003). Nevertheless, it should be noted that there was considerable scattering and overlapping of the MET values recorded in the 2 groups (Figure 3). With respect to LGE measurement, a significant, although weak, inverse correlation was found between the percentage of myocardium showing LGE and the MET values reached (r=-0,21; P=.044).
Figure 3. Differences in functional capacity (METs) between patients showing late gadolinium enhancement on magnetic resonance imaging (LGE+) and those without late gadolinium enhancement (LGE-) (significance level with the Student t test is indicated).
Multivariate Analysis
Multivariate analysis was performed in the subgroup of patients for which NT-proBNP concentrations were available, (71 patients), including the independent variables age, sex, history of atrial fibrillation (paroxysmal, persistent, or permanent), fibrosis, plasma NT-proBNP concentrations, maximum ventricular wall thickness (mm), and significant LVOT gradient (>30 mm Hg). The baseline characteristics of this subgroup of patients were similar to those of the total population studied. Among the variables included, only age, a history of atrial fibrillation, fibrosis on gadolinium-enhanced CMR, and the logarithm of NT-proBNP concentrations was independently associated with MET values on exercise testing (Table 4) (model r2=0.47; corrected r2=0.44).
DISCUSSION
The population studied presented severe disease in terms of functional capacity and risk factors for sudden death (52% presented 2 or more risk factors), as well as fibrosis on CMR (68.4% of patients presented areas of LGE). This degree of disease severity is comparable to findings from other studies (fibrosis on CMR imaging observed in 48%-81% of patients)9-11,14 and is characteristic of populations attended for HCM in referral centers.4
This study supports the concept that myocardial fibrosis, determined in vivo by LGE in CMR images, contributes to the functional limitations of patients with HCM independently of other factors, such as ventricular wall thickness, LVOT gradient, or atrial fibrillation. The statistical association was quite weak, however. Even when the analysis was restricted to the most significant group of variables (atrial fibrillation, age, NT-proBNP concentrations, and fibrosis), only 47% of the MET variability could be explained. This difficulty for explaining the functional limitations in HCM is consistent with published results7,8,19 and reflects the complex and heterogeneous nature of the processes implicated, which include diastolic dysfunction, systolic dysfunction, LVOT obstruction, mitral regurgitation, and ischemia, in addition to the numerous extracardiac factors that affect functional capacity. In keeping with other authors, we found that logNT-proBNP was the best predictive factor for exercise capacity (r=-0.5).8 Nevertheless, the fact that other parameters (eg, LVOT obstruction, left ventricular ejection fraction, and wall thickness) did not attain consideration as significant independent predictors of functional capacity, in contrast to other studies,6,19-24 indicates that the results should be interpreted with caution. One peculiar characteristic of our series was the small representation of patients with depressed ejection fraction (only 6 [6.2%] patients presented ejection fraction <50%). Because of this fact, we were unable to clarify the role of systolic function in exercise capacity or in relation to fibrosis.
The relationship between fibrosis and certain markers of disease severity have been described previously.1,10,14,25 Study of LGE on CMR imaging provides the possibility to determine this relationship in vivo. Fibrous tissue is seen in more severely diseased myocardium, which shows distensibility problems, greater thickening, poorer contractility, and a tendency to progressive dilatation, all of which lead to hemodynamic deterioration in the patient.9,11,12,26 Dumont et al10 found a relationship of LGE with the ischemic response on exercise testing, as well as with systolic dysfunction and a reduced capacity for increasing the LVOT gradient during exercise. These findings may help to explain the poorer functional capacity of patients presenting LGE.
Of note in our study, LGE measurement was not a better predictor of functional capacity than simple differentiation between the presence or absence of LGE. This indicates that LGE actually reflects several types of histologic anomalies having different prognostic significance (diffuse increase of the interstitial matrix, architectural structure loss, plexiform fibrosis at the interventricular septal insertion sites, perivascular fibrosis, and areas of scarring16). Currently, there is no consensus on the most suitable method for measuring LGE. Apart from quantification based on the threshold signal that we used,9 other groups have used measurement by planimetry,11,14 dispersion of the myocardial signal intensity,26 and counts of the number of segments with LGE.10 Moreover, in contrast to what occurs in processes such as myocardial infarction, the areas showing LGE in HCM patients, particularly those with diffuse myocardial injury, are usually poorly delimited, present differing grades of involvement, and contain myocytes coexisting with abundant interstitial matrix at varying proportions.
All in all, it remains to be clarified whether some of the quantitative parameters used are ideal for determining the severity of the disease. Moon et al11 have even suggested that a qualitative assessment, indicating the distribution pattern of fibrosis, may provide additional prognostic information.
Although it was not part of the main purpose of our analysis, a statistical association was found in our population between LGE on CMR imaging and the predictors of sudden death used in clinical practice, a finding that confirms the results of other groups4,10-12,14 and supports the probable role of myocardial fibrosis in the origin of ventricular arrhythmia and sudden death in these patients (data not shown).
Limitations
This is an observational study performed in an unselected patient population referred to the specialized participating clinics; therefore it is subject to potential biases and interactions among the different variables studied. In addition, the population characteristics were typical of those seen in specialized HCM clinics, with a more severe profile of the disease than that of the overall population of HCM patients.
We observed a significant, although modest, contribution of fibrosis to explain the functional deterioration in the patients studied. In any case, the complexity of this cardiac condition and the numerous mechanisms implicated makes investigation into only one of these mechanisms difficult. We cannot exclude that the involvement of other variables in the origin of dyspnea might have been clarified in a larger patient population.
The present study has a purely cross-sectional design. Long-term follow-up of our patients would likely provide additional information, such as the value of fibrosis for predicting progression to systolic dysfunction, ventricular arrhythmia, or the development of atrial fibrillation.
Lastly, determination of oxygen consumption during exercise would have allowed more reliable and reproducible quantification of the patients' functional capacity.19,27
CONCLUSIONS
Late gadolinium enhancement and its measurement (percentage of enhanced tissue) in patients with HCM are associated with poorer exercise capacity, estimated by treadmill exercise testing. The correlation is statistically weak, but is independent of other predictors associated with functional deterioration in these patients.
ABBREVIATIONS
CMR: cardiac magnetic resonance
HCM: hypertrophic cardiomyopathy
LGE: late gadolinium enhancement
LVOT: left ventricular outflow tract
NSVT: nonsustained ventricular tachycardia
Correspondence: Dr. F. Marín.
Servicio de Cardiología. Hospital Universitario Virgen de la Arrixaca.
Ctra. Madrid-Cartagena, s/n. 30120 El Palmar. Murcia. España.
E-mail: fcomarino@hotmail.com
Received January 19, 2008.
Accepted for publication April 16, 2008.