Keywords
INTRODUCTION
Hypertrophic cardiomyopathy (HCM) is a progressive disease characterized by inappropriate myocardial hypertrophy and, at the microscopic level, focal myocardial fibrosis, scar tissue, myofibrillar disarray, and small-vessel disease, leading to a heterogeneous clinical, and pathological profile.1 In necropsy studies, the severity of HCM has been related to the presence and amount of myocardial fibrosis.2-5 However, the prognostic value of myocardial fibrosis has been difficult to determine due to the lack of diagnostic methods able to detect its presence in vivo.
Until now, cardiac magnetic resonance imaging (MRI), with delayed enhancement (DE) following the administration of contrast material, is the only approach that allows in vivo detection of myocardial scarring caused by necrosis,6 as well as focal myocardial fibrosis of nonischemic origin.7,8 Several authors9 have recently shown the potential value of this technique in the clinical assessment of patients with HCM. Nevertheless, only a few studies have attempted to show the clinical value of DE in HCM. Our purpose was to describe the presence and distribution of myocardial fibrosis in a series of patients with HCM, and to determine whether there is a relationship between the extent of myocardial fibrosis and the severity of the disease.
METHODS
Patients
The study included 43 consecutive patients (30 men; mean age, 47 [18] years) diagnosed with HCM on echocardiography, defined as left ventricular wall thickness ≥15 mm, with no known cause of ventricular hypertrophy. Contraindication for the cardiac MRI study was the only exclusion criterion. Clinical risk was assessed using already established criteria10: family history of sudden death, inexplained syncope, documented ventricular tachycardia, severe left ventricular hypertrophy ≥30 mm.
Cardiac MRI Studies
All patients underwent cardiac MRI with contrast material using a Philips Intera 1.5T scanner. After the usual scout views were obtained, cine-MRI was performed with a steady-state free-precession sequence in slices along the longitudinal axis of the left ventricle, as well as multiple 10-mm slices along the transverse axis from the base to the apex of the left ventricle. At least 16 phases of the cardiac cycle were acquired for each slice and reproduced as a continuous loop.
An endovenous injection of 0.1 mmol/kg of gadoteridol (Gadovist, Schering AG, Berlin, Germany) was given, and a 3D gradient-echo inversion-recovery sequence was acquired 10 minutes after injection of the contrast material11 to analyze DE. The inversion time was adjusted to suppress the normal myocardium signal (200-300 ms). This sequence was programmed in multiple slices of the transversal axis of the left ventricle, using the same orientation as for cine-MRI imaging.
Image Analysis
The cine-MRI and DE images were analyzed in a second stage with special software (Mass, MEDIS, Leiden, The Netherlands), and the endo- and epicardial borders of the left ventricle were manually traced on the systolic and diastolic cine-MRI images for each transversal section. The left ventricular mass (LVM), end-diastolic volume, and end-systolic volume were calculated for each patient.
The presence of DE in the left ventricle was visually analyzed, along with its distribution pattern, and location. Based on the images obtained from the transverse axis of the left ventricle, planimetry was used to calculate the total weight of myocardial fibrosis identified by DE.
Statistical Analysis
Continuous quantitative variables are expressed as mean (standard deviation) and compared using the Student t test for independent samples. The Mann-Whitney U-test was used for variables that did not have a normal distribution. A P value less than .05 was considered significant.
RESULTS
The clinical characteristics of the patients studied are shown in Table. Cardiac MRI showed left ventricular hypertrophy with a maximal wall thickness of 14-39 mm (mean, 21 [6] mm), and an LVM of 67-358 g (mean, 169 [61] g). All patients except 2 showed LVEF values on cardiac MRI ≥50% (mean, 71% [13%]).
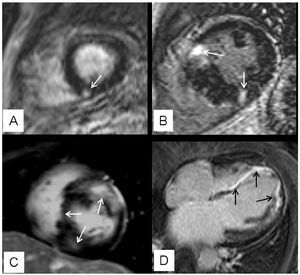
Delayed enhancement was observed in 63% (27/43) of patients, with the following distribution patterns: a) unifocal, affecting different regions of the left ventricular myocardium (4 patients; 15%); b) bifocal, affecting the areas where the LV and RV join (10 patients; 37%); c) multifocal (10 patients; 37%); and d) diffuse (3 patients; 11%) (Figure 1). The total calculated mass of myocardial fibrosis was between 1 and 59 g (mean, 17 g).
Figure 1. Distribution patterns of focal myocardial fibrosis detected by DE. A: corresponds to a unifocal pattern which, in this case, affects the inferior septum (arrow); B: shows the characteristic bifocal pattern affecting the area where both ventricles join (arrows); C: corresponds to a multifocal patterns that includes segments other than the left ventricle (arrows); D: illustrates an example of a diffuse pattern with a long extension of DE (arrows).
A good correlation was observed between the amount of myocardial fibrosis and the degree of hypertrophy in terms of maximal wall thickness (r=0.58; P=.002), as well as with the LVM (r=0.60; P=.001) (Figure 2). When considering the patient group with and without DE, we found significant differences in maximal wall thickness (23 [7] vs 18 [4] mm, respectively, P=.045). However, there were no significant differences in LVM (176 [71] vs 158 [38] g, P=.55), or end-diastolic volume (133 [46] vs 116 [27] mL; P=.38), or end-systolic volume (43 [35] vs 30% [10%]; P=.22).
Figure 2. Plots showing the correlation between myocardial fibrosis, detected by DE, with LVM (A) and with maximal wall thickness (B).
A higher prevalence of familial cases was found among patients with myocardial fibrosis compared to patients without myocardial fibrosis (48% vs 13%, respectively; P=.08). Differences were also found when comparing the presence of conventional risk factors in both groups: 13 patients (48%) with myocardial fibrosis proven by DE presented at least 1 risk factor, whereas only 2 (13%) patients without myocardial fibrosis had one of these risk factors (P=.04) (Figure 3).
Figure 3. Comparison of the number of clinical risk factors between the group with and the group without DE. Most patients without DE did not present any clinical risk factor, whereas almost half the patients with DE presented at least one clinical risk factor.
DISCUSSION
This study shows that on cardiac MRI, most patients with HCM present DE indicative of focal myocardial fibrosis. In keeping with reports recently published in the literature,9,12 this study found a correlation between the amount of myocardial fibrosis and the degree of hypertrophy by cardiac MRI. However, despite the relationship between the extension of DE and LVM, or maximal wall thickness, it is worth noting the possible absence of myocardial fibrosis even in cases with an obvious increase in LVM.
Varnava et al2 demonstrated in a histological study of patients with HCM, whether deceased or subject to heart transplantation, that the presence of myocardial fibrosis in HCM is related to the degree of hypertrophy. Although these observations are based on a group of patients with much more advanced disease, the findings of our study show myocardial fibrosis is also observed in an unselected population of patients with HCM, as found in other studies.9,12 Additionally, myocardial fibrosis was present in segments with greater hypertrophy.
The importance of detecting myocardial fibrosis in unselected patients with HCM lies in the potential prognostic implication of this finding. A combination of the different clinical risk factors is currently used to stratify the risk of patients with HCM. However, the identification of patients with HCM and a high-risk profile continues to be a challenge. Moon et al9 observed that the extension of the DE helped identify patients with a high-risk profile. Moreover, a greater extension of DE was observed in patients with dilation and progressive left ventricular dysfunction. In our study, the consecutive inclusion of patients resulted in a population with a relatively benign profile, since all patients except for one had none, or one risk factor for sudden death. When comparing these 2 groups, we observed that the absence of DE is more commonly associated with patients with no risk factors than those with a risk factor. This finding is of particular interest, especially when considering that there is still some debate in primary prevention about automatic implantable defibrillators for patients with only one risk factor.13 Based on the literature published to date, however, the prognostic significance of DE in these patients is still not determined. Studies with long-term follow-up and large populations are still required to establish if DE in patients with only one risk factor for sudden death will change the therapeutic approach in primary prevention.
It is important to mention that the 2 patients in our series who had progressive HCM with severe left ventricular dysfunction presented greater myocardial fibrosis (Figure 1D). In these 2 patients, the total myocardial fibrosis mass was 58 and 59 g, whereas in the remaining patients it was from 1 to 48 g. This observation also correlates with the anatomical pathology findings, which showed that patients with HCM who evolved rapidly to progressive heart failure presented extensive myocardial scarring of the left ventricle at the time of heart transplantation or autopsy.14
In this study, the more frequent distribution pattern for DE was bifocal, affecting the areas where the 2 ventricles were joined, and multifocal. The bifocal pattern has been previously related to a benign profile.9,12 However, the size of our sample is not sufficient to draw conclusions regarding the potential prognostic implications for the various distribution patterns of DE.
This study is limited by its cross-sectional design, which makes it impossible to establish a relationship between myocardial fibrosis and HCM prognosis. Studies with long-term follow-up are needed to draw conclusions, as well as to assess the potential prognostic value of the various distribution patterns for myocardial fibrosis.
In conclusion, this study shows that focal myocardial fibrosis, identified by DE in the cardiac MRI, is a common finding in patients with HCM. Although myocardial fibrosis is not necessarily observed in all patients with more hypertrophied ventricles, when present there is a correlation between the amount of myocardial fibrosis and the degree of hypertrophy. In addition, DE in HCM tends to be more prevalent among familial cases, as well as among those with a potentially higher risk profile. Confirmation of this in future studies may confirm the value of DE as a risk factor in HCM.
ABBREVIATIONS
HCM: hypertrophic cardiomyopathy
MRI: magnetic resonance imaging
DE: delayed enhancement
LVM: left ventricular mass
See editorial on pages 1-4
Correspondence: Dra. Sandra Pujadas.
Vía Augusta, 17-19, sobreático 3.ª. 08006 Barcelona. España.
E-mail: spujadas@santpau.es
Received April 12, 2006,
Accepted for publication July 13, 2006.