Keywords
INTRODUCTION
The priority in primary prevention of cardiovascular diseases (CVDs) is to control risk factors, and the choice of therapeutic approach will depend on an appropriate stratification of the risk of developing such diseases.
Systemic hypertension (HT) and dyslipidemia, particularly hypercholesterolemia, are the main determinants of CVD risk in Spain and the rest of the world. The risk, according to population-based estimates, of suffering ischemic heart disease is significantly greater for hypercholesterolemia than for HT. However, for cerebrovascular disease, HT is the main determinant. In a recent publication, Medrano et al1 analyzed the risk attributed to different risk factors for developing ischemic heart disease: smoking and excess body weight are those with greatest population-based risk, while HT and hypercholesterolemia have a similar risk ratio, although HT is more important in men and hypercholesterolemia in women.
In each individual, management of risk factors is necessary in order to estimate the patient's cardiovascular risk. Several risk equations are available, and these include systolic blood pressure (SBP) and plasma cholesterol concentration. The SCORE tables2 and those proposed by the HT Guidelines of the European Societies for Cardiology and Hypertension3 should be our reference for estimating this risk and the approach and therapeutic goals. In Spain, the VERIFICA study4 concluded that the risk equation adapted to our population from the original Framingham tables is valid, as it shows good accuracy and reliability for predicting ischemic heart disease at 5 years in a population group aged between 35 and 74 years.
All recommendations indicate that a general strategy for control of risk factors will be preferred and that the therapeutic goals, the threshold for initiating treatment, and choice of drugs will depend on the general risk.5 However, when deciding on pharmacologic interventions for risk factors (in this case, for HT and hypercholesterolemia), in order to prevent the first cardiovascular complication or renal disease, the balance between sensitivity and specificity of the accepted cut-points should be taken into account in the decision to start therapy; we should also remember that this cut-point is relatively arbitrary. Thus, to achieve a sensitivity of 100% for identifying a group of individuals who will develop cardiovascular disease over a 10-year period, we would need to treat the entire population, and even so, we would not succeed in preventing all events as the efficacy of antihypertensive agents and lipid-lowering drugs is limited. At this point, and in Spain, it is important to bear in mind the results of the VERIFICA study,4 in which, with the observed incidence of cardiovascular diseases, every 1-unit increase in sensitivity is equivalent to identifying 1.8 individuals who will develop coronary artery disease, and every 1-unit increase in specificity would identify 55 who are not at risk.
PHARMACOLOGICAL TREATMENT OF HYPERTENSION
Considerations for Drug Selection
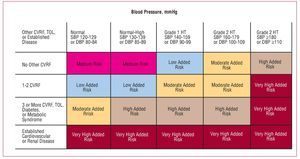
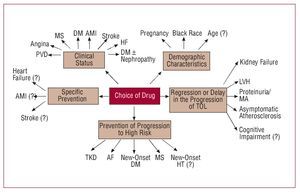
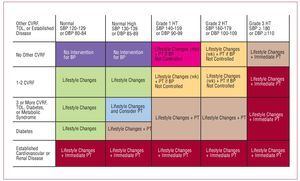
The recent HT guidelines published by the European Societies for Cardiology and Hypertension3 include a proposal for risk stratification based, on the one hand, on blood pressure and, on the other, on the presence of other cardiovascular risk factors (CVRF), diabetes, diagnosis of metabolic syndrome, target organ lesion, and clinical cardiovascular disease (Figure 1). In line with this proposal, a decision needs to be made regarding the moment when pharmacological treatment is started, the blood pressure target, and the choice of the most appropriate agents. Together, the targets of antihypertensive treatment should include: a) strict control of BP in most patients; b) control of associated risk factors; c) prevention, regression, or delay in the progression of lesions in the target organ; d) prevention or delay in the transition to high cardiovascular risk; and e) choice of drugs according to patient characteristics and nonhypotensive properties. Figure 2 is a flow diagram of the considerations to be borne in mind when selecting the most appropriate pharmacologic strategy in a given patient; in addition to the antihypertensive efficacy and the profile of adverse effects of the drugs, specific patient and drug characteristics should be considered.
Figure 1. Proposal for risk stratification of the European Societies of Hypertension and Cardiology. Based on Mancia et al.3 CVRF indicates cardiovascular risk factor; DBP, diastolic blood pressure; HT, hypertension; SBP, systolic blood pressure; TOL, target organ lesion.
Figure 2. Considerations to bear in mind when selecting the most appropriate pharmacologic strategy. AF indicates atrial fibrillation; AMI, acute myocardial infarction; DM, diabetes mellitus; HT, hypertension; LVH, left ventricular hypertrophy; MA, microalbuminuria; MS, metabolic syndrome; PVD, peripheral vascular disease; TKD, terminal kidney disease; TOL, target organ lesion. Based on Mancia et al.3
To date, we do not have a treatment for the underlying HT disease, this is only possible in some cases of secondary HT; in addition, the possibility of selecting treatment according to the pathophysiologic characteristics of the patients is limited. Differences in hemodynamic and neurohormonal patterns, autonomic tone, or changes in central nervous system activity and, to a lesser degree, the genetic profile cannot be easily applied in clinical practice for selecting antihypertensive therapy.
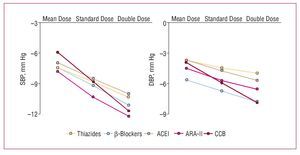
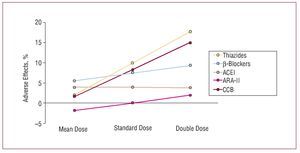
Although, in general, there do not appear to be large differences in the efficacy of the different families of drugs at lowering blood pressure, something which depends mainly on the dose used, the adverse effect profile depends both on the type of drug and its dose. In a metaanalysis published recently by Law et al,6 who analyzed the findings of 354 randomized clinical trials, a direct relationship was found between the dose of the drugs and their hypotensive efficacy for angiotensin II receptor antagonists (ARA-II) and calcium channel blockers in the case of SBP, and for ARA-II, calcium channel blockers, and b-blockers in the case of diastolic pressure (Figure 3). In contrast, the incidence of adverse effects did not increase significantly on increasing the dose of thiazide diuretics and calcium channel blockers, while the increase was smaller or, at times, undetectable for ARA-II and angiotensin converting enzyme (ACE) inhibitors, which were the drugs associated with the lowest incidence of adverse effects at the initial dose (Figure 4).
Figure. 3. Mean decrease in systolic blood pressure (SBP) over 24 hours in 354 randomized trials (40 000 patients on active treatment and 16 000 on placebo) that compared standard doses with half dosing and double dosing. ACEI indicates angiotensin converting enzyme inhibitors. ARA-II, angiotensin II antagonists; BB, b-blockers; CCB, calcium channel blockers. Based on Law et al.6
Figure 4. Adverse effects of drugs in 354 randomized trials (40 000 patients on active treatment and 16 000 on placebo) that compared standard doses with half dosing and double dosing. ACEI indicates angiotensin converting enzyme inhibitors; ARA-II, angiotensin II antagonists; BB, b-blockers; CCB, calcium channel blockers. Based on Law et al.6
In short, and in accordance with recent clinical practice guidelines, when selecting antihypertensive drugs, whether in monotherapy or in combination, we should take into account, among other things, the possible previous exposure of patients to a certain class of drugs, their effect on other risk factors that might be associated with HT, the presence or absence of lesions in the target organs, CVD (an important differential factor between different drugs), presence of renal disease and/or diabetes, other diseases that might influence the use of a certain group of drugs, cost, efficacy throughout the day, efficacy with a single daily dose, and profile of adverse effects, which, as mentioned, clearly varies between drugs.
Benefits of Lowering Blood Pressure
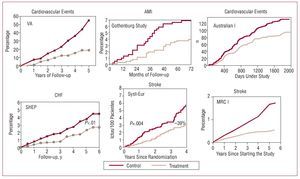
The relationship between pharmacological reduction in BP in hypertensive individuals and the decrease in risk of suffering cardiovascular and renal complications has been known for more than half a century. In addition, the improved prognosis has been observed for short follow-up periods in a large number of clinical trials, both for overall cardiovascular complications and for reduction in risk of stroke, heart failure, and ischemic heart disease, and this is directly related to the decrease in BP levels. Indeed, the decrease in BP is the determining factor, although for the same hypotensive effect, the decrease in the risk of stroke is larger than the decrease in the risk of ischemic heart disease7-12 (Figure 5). On this point, the results of the VALUE study should be highlighted.13 This study included high-risk hypertensive patients in whom, during the first 6 months of follow-up, differences in cardiovascular risk were observed (in particular, risk of myocardial infarction) between the patients randomized to receive valsartan or amlodipine treatment, although these differences disappeared at the end of follow-up. This behavior can be explained by a larger initial reduction in BP in patients treated with amlodipine and emphasizes the importance of achieving good BP control in a short time, at least in high-risk patients with HT.
Figure 5. Early clinical benefit of antihypertensive treatment. AMI indicates acute myocardial infarction; CHF, congestive heart failure. Based on several sources.7-12
Antihypertensive therapy should be started in accordance with the recommendations of the clinical practice guidelines.3 In low-risk and moderate-risk individuals, lifestyle changes should be tried for months or weeks and, after confirming that high BP persists, pharmacotherapy should be started in high- or very high-risk hypertensive patients. In such patients, it is preferable to start therapy with a combination of 2 antihypertensive agents at low doses and then make adjustments according to the course of BP (Figure 6).
Figure 6. Initial antihypertensive treatment. CVRF indicates cardiovascular risk factors; DBP, diastolic blood pressure; TOL, target organ lesion; SBP, systolic blood pressure. Based on Mancia et al.3
A larger decrease in BP seems to be associated with a lower cardiovascular risk, at least in hypertensive patients at greatest risk. A number of studies have been published addressing this point, particularly in patients with diabetes. The results of a metaanalysis that deal with this topic indicate that a difference in BP of 6/4.6 mm Hg is accompanied by an additional 36% reduction in the risk of stroke, and reductions of 31% in the risk of heart failure, 33% in cardiovascular mortality, and only 16% in ischemic heart disease.14 These results have been confirmed by the HOT study,15 and form the basis for recommending a BP goal in patients with diabetes of less than 130/80 mm Hg, which in practice demands the use of antihypertensive therapy in almost all patients. Thus, many recent clinical trials have confirmed the benefit of reducing the risk of cardiovascular complications using pharmacologic therapy in patients with BP below the actual definition of HT (<140/90 mm Hg). Almost all have been performed in high-risk patients with established cardiovascular disease or diabetes. Attaining PB levels of less than 130 (124-129)/80 (74-78) mm Hg is associated with a significant reduction in the risk of cardiovascular complications.3
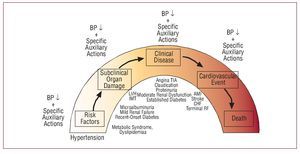
Patients with renal and cardiac damage (in particular, microalbuminuria and left ventricular hypertrophy, assessments that should be included in the initial examination of all hypertensive patients) are at higher cardiovascular risk and so it is recommended to include these patients in the high-risk group. They should therefore have the same BP treatment targets as patients with diabetes. The rationale behind this approach, at least in patients with renal damage, is the existence of a relationship between hypotensive effect and decrease in urinary excretion of albumin. Figure 7 presents schematically the proposal for ongoing cardiovascular treatment, in which different phases of the course of vascular and renal disease associated with HT are included and the impacts of pharmacological reduction of BP are indicated along with the characteristics of the antihypertensives themselves with their relative and increasingly smaller effect on the progression of the disease and its prognosis.16
Figure 7. Proposed spectrum of cardiovascular treatment, in which different phases of the course of vascular and renal disease associated with HT are included and the impact of pharmacological reduction of blood pressure are indicated along with the characteristics of the antihypertensives themselves with their relative and ever diminishing impact on the progression of the disease and its prognosis. AMI indicates acute myocardial infarction; BP, blood pressure; CHF, congestive heart failure; IMT indicates intimalmedial thickness; LVH, left ventricular hypertrophy; RF, renal failure. Based on Zanchetti.16
Benefits of Antihypertensive Treatment in Patients With Initial BP Less Than 140/90 mm Hg
The new HT guidelines include the concept of threshold and flexible BP goal according to the baseline cardiovascular risk of the patients. In high-risk patients, including diabetic patients, patients with metabolic syndrome, and hypertensive patients with target organ lesion, the goal is to achieve BP<130/80 mm Hg, and the threshold for diagnosis of HT is set at 130/85 mm Hg. In a number of recent clinical trials that included high-risk patients with baseline BP less than 140/90 mm Hg, an improvement in prognosis was observed associated with a larger reduction in BP, which in all cases attained mean BP of less than 130/80 mm Hg.17. We should highlight the results of the MICROHOPE,18 ADVANCE,19 and ABCD20 trials, which included diabetic patients treated with ACE inhibitors, usually in combination with thiazide diuretics or calcium channel blockers. These results indicate that, in high-risk hypertensive patients, and in diabetic patients in particular, the BP goal should coincide with that recommended by the guidelines (<130/80 mm Hg), as there is no evidence of a J curve. Such a curve has only been reported in patients with ischemic heart disease in whom the diastolic pressure after treatment is less than 70 mm Hg.21 Thus, the recent results of the arm of dual blockade (ramipril plus telmisartan) in the ONTARGET study,22 which included patients at high cardiovascular risk—mostly hypertensive patients with a history of chronic ischemic heart disease, could be influenced by a high incidence of hypotension during follow-up forcing withdrawal of the study medication.
Selection of the Initial Antihypertensive Agent
As mentioned, in clinical practice the hypotensive efficacy, safety, and cost, or, in short, the cost-effectiveness ratio, are the main elements to take into account when starting and maintaining pharmacotherapy. Although with certain individual variations, in clinical practice, and perhaps with the exception of b-blockers for treatment of isolated systolic HT in elderly patients, there is a relative equivalence in the antihypertensive action of the pharmacological groups available, although differences in the adverse effect profile do bear a dose-dependent relationship. In many cases, the clinical practice guidelines recommend starting treatment with fixed combinations at low doses as, in addition to the hypotensive synergy, the appearance of adverse effects is limited.
With very few exceptions, the clinical trials available indicate that reducing BP is the main determinant in the prognosis of the hypertensive patients treated and that choice of antihypertensive agent is a minor determinant. It is true that during the first years of treatment, which includes the observation period of clinical trials, the decrease in BP is, in itself, of primary importance and determines the prognosis of the patients. However, it may be that in the long term, the different actions of the drugs other than their effect on BP take on greater clinical relevance. Moreover, it may be that persistence during treatment of BP levels within the range of hypertension hinders the clinical expression of these effects of the drugs.
Currently, as an alternative to first-line treatment, we have 5 families of antihypertensive drugs, and the first component of a sixth family may be added in the near future. Diuretics, b-blockers, calcium channel blockers, ACE inhibitors, and ARA-II form the therapeutic arsenal available for pharmacologic treatment of HT. It is possible that towards the end of 2008 we will also have available the first drug (aliskiren) to block the action of renin. This drug has been shown to have a hypotensive efficacy similar to that of other drug groups with a good tolerability profile and persistence of its antihypertensive effect in the long term.23 a-blockers (specifically doxazosin), after the findings of the ALLHAT study,24 are no longer considered as first-line therapy. Also, according to the results of that study, the American Joint National Committee guidelines (JNC-8) indicate that a low-dose thiazide should be preferred as the initial treatment in most hypertensive patients.25
In view of the impact of the ALLHAT study on clinical practice guidelines, we will make a specific comment here. ALLHAT is the largest study carried out in patients with HT and is sponsored by the American National Heart, Lung, and Blood Institute. It was designed to assess the incidence of coronary deaths or nonfatal myocardial infarction in high-risk hypertensive patients treated with a strategy based on calcium channel blockers (amlodipine), an ACE inhibitor (lisinopril), or an a-blocker (doxazosin), compared to a thiazide diuretic (chlorthalidone). A previous publication showed that chlorthalidone was superior to doxazosin (the differences were based on the reduction in risk of heart failure), and this led to the early discontinuation of the doxazosin arm.26 Secondary outcome measures included overall mortality, stroke, and other CVDs. The study was powered to detect differences between antihypertensive drugs, as it included between 9000 and 15 000 hypertensive patients per treatment group, and these patients were followed for a long period (4.8 years). No significant differences were observed between the different groups for the primary outcome measure: compared to diuretics (11.5% of patients with any of the complications of the primary outcome measure), the relative risk (RR) was 0.98 (95% confidence interval [CI], 0.90-1.07) for amlodipine and 0.99 (95% CI, 0.91-1.08) for lisinopril. Likewise, no significant differences were observed in overall mortality in the 3 treatment groups. In comparison with the diuretic, SBP after 5 years of treatment was significantly greater in the amlodipine group (0.8 mm Hg; P=.03) and lisinopril group (2 mm Hg; P<.001) and diastolic blood pressure after 5 years was significantly greater with amlodipine (0.8 mm Hg; P<.001). Comparing the components of the secondary outcome measure among groups of amlodipine- or chlorthalidone-treated hypertensive patients, a significant effect was only observed in the incidence of heart failure at 6 years of follow-up in the amlodipine-treated group (10.2% vs 7.7%; RR=1.38; 95% CI, 1.25-1.52). Comparing the groups treated with lisinopril and chlorthalidone, ACE inhibitors were associated with a greater risk of cardiovascular complications after 6 years of follow-up (33.3% vs 30.9%; RR=1.10; 95% CI, 1.05-1.16), greater incidence of stroke (6.3% vs 5.6%; RR=1.15; 95% CI, 1.02-1.30), and heart failure (8.7% vs 7.7%; RR=1.19; 95% CI, 1.07-1.31).
The investigators of the ALLHAT study concluded that thiazide diuretics (chlorthalidone) should be considered as the first therapeutic alternative in the treatment of HT. Despite the strong influence of the ALLHAT study on clinical practice, we should bear in mind certain considerations when interpreting the results. The characteristics of the study design did not allow the hypertensive patients included in each of the therapeutic modalities studied (chlorthalidone, doxazosin, amlodipine, and lisinopril) to receive drugs from any of the other groups studied. Possible allowed combinations were use of b-blockers, clonidine, reserpine, and hydralazine, which are not common in clinical practice. In addition, the differences in BP between the different patient groups during follow-up could have influenced the results; the reduction in SBP among patients randomized to receive diuretic was greater than the general reduction observed at the end of the study and may have been responsible for lower cardiovascular risk. The differences in incidence of heart failure between the diuretic group and the other treatment modalities are noteworthy. A number of aspects should be considered when interpreting this observation; for example, cases of heart failure were significantly more frequent in this study than in other similar studies and, in addition, the diagnostic criteria taken into consideration were not reported. However, the cases of fatal heart failure or heart failure requiring admission to hospital were similar in both the lisinopril and chlorthalidone groups and significantly more frequent in the group of patients treated with amlodipine and, in particular, doxazosin. The characteristics of the population of hypertensive patients included in the ALLHAT study might also have influenced the outcomes in the group treated with lisinopril. More than 35% of the patients were black, a fact that, along with poorer SBP control, might be responsible for the lower efficacy of ACE inhibitor treatment. In addition, the hypertensive black patients treated with lisinopril who did not achieve the BP goal defined in the ALLHAT study received treatment with b-blockers, and this combination is probably no longer the best combination in this group of patients.
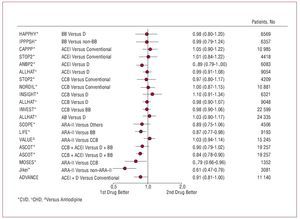
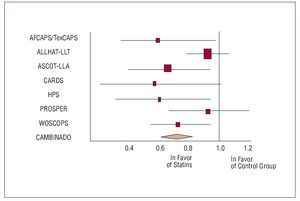
Figure 8 shows the results of the main clinical trials for HT that help has to tailor the therapeutic decisions.27 Special comment should also be made for the results of the LIFE28 and ASCOT29 trials, as they bring into question the efficacy of the combination of 2 classic antihypertensive agents (b-blocker and thiazide) compared to other combinations (losartan and thiazide in the LIFE study and perindopril and amlodipine in the ASCOT trial). In both clinical trials, the treatment arms that did not include b-blockers (atenolol in both studies) were accompanied by better cardiovascular prognosis. In the LIFE study, which included more than 9000 hypertensive patients with left ventricular hypertrophy in the ECG and a mean follow-up of 4.8 years, treatment with losartan and thiazide was accompanied by a 13% reduction in risk of occurrence of one of the components of the primary outcome measure (cardiovascular death, myocardial infarction, and stroke) and this decrease was 25% for the case of stroke (fatal or nonfatal); this behavior cannot be explained by the minimal differences in BP (145.4 mm Hg and 144.1 mm Hg, and 81.3 mm Hg and 80.9 mm Hg for systolic and diastolic blood pressures in the losartan and atenolol groups, respectively) during follow-up. The ASCOT study included 19 257 hypertensive patients with high cardiovascular risk—defined as the coexistence in the same patient of at least 3 other risk factors—who were randomized to receive amlodipine (5-10 mg/d) in combination with perindopril (4-8 mg/d) for control of BP or atenolol (50-100 mg/d) in combination with a thiazide (1.25-2.5 mg/d of bendroflumethiazide) and potassium when necessary. The primary outcome measure of the study was a composite of nonfatal (including silent) myocardial infarction and death due to ischemic heart disease. The study was discontinued early after a mean of 5.5 years of follow-up. Although not statistically significant, a smaller incidence of components of the primary outcome measure was observed in patients treated with amlodipine, corresponding to a reduction of 10% in the relative risk (P=.1052); however, a highly significant reduction in the episodes of fatal and nonfatal stroke, the set of cardiovascular and interventional complications, and overall mortality were observed. Likewise, a 30% reduction in the appearance of new-onset diabetes was observed. As in the LIFE study, the minimal differences in BP control during follow-up could not fully explain the results and, after adjustment for variables that might have influenced prognosis, only 40%-50% of the differences observed could be accounted for.
Figure 8. Trials with different antihypertensive treatments. Primary outcome measures (relative risk [95% confidence interval]). AB indicates a-blockers; ACEI, angiotensin converting enzyme inhibitors; ARA-II, angiotensin II antagonists; BB, b-blockers; CCB, calcium channel blockers. Based on Mancia.27
The results of the ASCOT-CAFÉ substudy30 might help explain the differences in cardiovascular protection between amlodipine-based and b-blocker-based treatment; for the same level of brachial pressure in the 2 groups, the patients who received b-blockers showed a significantly higher central aortic pressure, with a mean difference in SBP of 4.3 mm Hg, mainly due to the so-called augmentation index (distance from the suprasternal notch for measurement of the central aortic waveform and the peak of the systolic pressure wave). In this group of patients, a multivariate analysis confirmed that the central aortic pressure was significantly associated with risk of cardiovascular and renal complications. The greater velocity of the aortic reflection waves in the group of patients treated with b-blockers seems to be the main determinant of this behavior.
Recently, the NICE (National Institute for Clinical Excellence) Group has published a metaanalysis that shows lower efficacy in terms of cardiovascular protection and a lower cost-effectiveness ratio for treatment with b-blockers, indicating that, after the results of the ASCOT29 and LIFE28 studies, they should be eliminated from first-line treatment of HT and reserved for indications where they are obligatory such as ischemic heart disease and, in particular, heart failure.31 However, it remains to be clarified whether this is a limitation of treatment with atenolol itself or whether other b-blockers with different hemodynamic, neurohormonal, and metabolic effects might have a greater impact on central aortic pressure and, therefore, have a similar cardiovascular and renal protection to that of new antihypertensive agents. In contrast, the European guidelines for HT maintain the b-blockers as first-line antihypertensive therapy, although they strongly advise against their combination with thiazides (Figure 9).3
Figure 9. Recommendations of the ESC and ESH guidelines concerning combination antihypertensive treatment. The preferred combinations for the general population of hypertensive patients are presented with thick lines. The boxes indicate the pharmacologic groups of proven efficacy in controlled clinical trials. Based on Mancia et al.3
The practical importance of this new situation in the treatment of HT, although key to a better understanding of the mechanisms by which cardiovascular disease occurrs, is limited in Spain as antihypertensive therapies based on b-blockers combined with thiazides are hardly used. However, we should be inclined to think that a modern combination of antihypertensive agents seems to be associated with better cardiovascular protection and to reduce morbidity and mortality associated with HT.
Special mention should be made of the results of interaction between antihypertensives and atorvastatin in the ASCOT (ASCOT 2´2).32 In that analysis, for a similar degree of BP control and modification of plasma lipids, the benefits of treatment with atorvastatin were modulated by the antihypertensive strategy used. The primary outcome measure of the study (composite of nonfatal [including silent] myocardial infarction and death due to ischemic heart disease) occurred in 53% fewer patients treated with the combination of atorvastatin and amlodipine compared to patients treated with amlodipine and placebo. In the group of patients randomized to atenolol, the combination of atorvastatin managed a 16% reduction in the occurrence of the primary outcome measure, although this difference was not statistically significant. Likewise, the benefits of the combination of amlodipine and atorvastatin were significantly greater for the other outcome measures, such as overall cardiovascular complications plus revascularization procedures, overall coronary events, and the composite endpoint of fatal and nonfatal stroke. In the group of patients treated with amlodipine, the combination of atorvastatin was accompanied by a highly significant decrease in the occurrence of the components of the primary outcome measure 90 days after starting the study through to the end of follow-up; however, in the group of patients randomized to the combination of atenolol and atorvastatin, no significant reductions were observed in cardiovascular complications during the entire follow-up.
A number of possible reasons can be put forward to explain these differing results, which are of great clinical relevance, because they indicate that it is possible to reduce cardiovascular risk in hypertensive patients by more than 50% with a relatively simple therapeutic intervention. This is the first time that such a large difference in benefit has been seen in a study that compares 2 treatment strategies. On the one hand, the greater effect on central aortic pressure of treatment with amlodipine might be amplified in presence of atorvastatin, and this may also enhance its effects on other mechanisms implicated in atherosclerotic vascular disease (in particular, endothelial dysfunction, oxidation of low-density lipoproteins [LDL], inflammation in the blood and vascular wall, etc). On the other hand, the combination of atenolol and thiazide might induce changes in carbohydrate metabolism and the lipid profile in general, and these may also have influenced the results.
Choice of Combination Therapy
Combined pharmacotherapy is essential for controlling BP in the vast majority of hypertensive patients. In most clinical trials, the mean number of drugs used was more than 3 even though a large proportion of patients did not manage to reach the ranges of normal BP. In the previous section, we have already commented on aspects related to the choice of combination therapy, and Figure 9 shows a schematic of main therapeutic combinations included in the European guidelines for HT. However, as for the choice of monotherapy, in addition to hypotensive efficacy, it is necessary to take into account how well tolerated the drugs are, the possibility of once-daily dosing, the demographic and clinical characteristics of the patients, etc. Initial combinations with a-blockers or b-blockers are not recommended, except with calcium channel blockers.
The combinations of ACE inhibitors and ARA-II with low-dose thiazide diuretics are one of the cornerstones of current antihypertensive therapy in view of their hypotensive efficacy and the variety of presentations available. However, the possibility that other types of drug combination, and in particular, ACE inhibitors or ARA-II plus calcium channel blockers or ACE inhibitors plus ARA-II, might be more effective for preventing cardiovascular and renal disease in hypertensive patients has always been debated. Recent results from 2 important clinical trials provide us with information of great practical relevance. In the ACCOMPLISH study,33 presented at the most recent meeting of the American College of Cardiology (Chicago, 2008) and still not published in full, combination treatment with an ACE inhibitor (benazepril) and calcium channel blockers (amlodipine) or a thiazide were compared in hypertensive patients. Despite similar control of BP during follow-up, the study was terminated prematurely due to greater prevention of cardiovascular complications in the group of patients randomized to the combination with amlodipine. Although a number of explanations can be put forward, in view of the results of the ASCOT-CAFÉ study,30 there may be differences in the decrease in central aortic pressure with the latter combination having greater efficacy. We should await publication of the definitive results for specific clinical implications, although it is very probable that this type of combination may become more relevant in the pharmacological treatment of HT.
In contrast, the results of the ONTARGET study,22 while not derived from a clinical trial in HT, advise against the use of dual blockade (ACE inhibitor plus ARA-II) for treatment of patients with high cardiovascular risk (75% with chronic ischemic heart disease, and almost 40% with diabetes). In that study, no differences were observed in the primary outcome measure (composite of cardiovascular death, myocardial infarction, stroke, and hospitalization for heart disease) among groups of patients randomized to ramipril (10 mg/d), telmisartan (80 mg/d), or both. The incidence of adverse effects, in particular episodes of hypotension, syncope, and renal dysfunction, was significantly higher in the combination group.
The results of the MOSES34 and Jikei-Heart35 studies reinforce the evidence available on the efficacy of adding a drug that blocks the renin-angiotensin-aldosterone system (eprosartan and valsartan, respectively) in hypertensive patients at high cardiovascular risk. In the case of the MOSES trial, patients with previous stroke were enrolled while the Jikei-Heart trial included a high proportion of hypertensive patients with ischemic heart disease. However, certain limitations in the study design should be taken into account when interpreting these results. In particular, the characteristics of the patients included, the open-label design, and the heterogeneity of the results are factors to take into consideration when translating the conclusions into clinical practice.
On the other hand, recent results of the ADVANCE study19 in patients with type 2 diabetes mellitus, most with a prior history of HT, support the initial use of combined therapy in high-risk patients. In that study, 11 140 patients with diabetes were randomized to receive a fixed combination of perindopril and indapamide or placebo added to conventional treatment. After a mean follow-up of 4.3 years, the patients assigned to receive the combination had a BP 5.6/2.2 mm Hg lower than that of the placebo group and a 9% reduction was observed in the occurrence of the primary outcome measure, a composite of macrovascular and microvascular complications (0.91; 95% CI, 0.83-1; P=.04). Despite the size of the effect of some of the study outcomes, the results suggest that, after 5 years of follow-up, only 1 death would be prevented for every 79 patients treated. The largest decrease in BP in the combination therapy group was, without doubt, the determining factor in the better prognosis.
Treatment of Hypertension in Elderly Patients
Pharmacological treatment of HT has been shown to be effective in preventing cardiovascular and renal complications. The clinical information available in the last decade confirms that the benefit is observed in different patient subgroups and with different classes of antihypertensives. However, as the clinical practice guidelines mention, the evidence available on the benefit of treating hypertensive patients aged over 80 years is not particularly strong. Thus, although there is a linear relationship between the increase in BP levels and the risk of stroke, this relationship is attenuated with age. Furthermore, epidemiological studies indicate that there is an inverse relationship between BP and mortality in those aged over 80 years, which indicates the possibility of greater risk of antihypertensive therapy in elderly patients or a relationship with clinical pictures associated with decreased BP (cancer, dementia, myocardial infarction, and heart failure).
A recent prospective study that included hypertensive patients aged over 80 years, 84.5% of whom received pharmacological treatment for HT, observed that after adjusting for variables that might influence prognosis, there was a decrease in survival in the subgroup of patients with SBP less than 140 mm Hg. The randomized studies that included elderly hypertensive patients excluded patients aged over 80 years or included so few patients that definitive conclusions could not be drawn. The results of a metaanalysis in this group of patients indicated that the reduction of 36% in the risk of stroke might be counteracted by a greater overall risk of mortality (increase of 14%; P<.05).36 The results of a pilot study (pilot phase of the HYVET study)37 coincide with those of the metaanalysis, that is, treatment of HT in those aged over 80 years might be associated with a decrease in the risk of stroke but an increase in overall mortality.
The recent publication of the final results of the HYVET study38 provides us with information of great clinical relevance. The study included 3845 hypertensive patients aged over 80 years with SBP greater than 160 mm Hg who were randomized to receive sustained-release indapamide (1.5 mg/d) or placebo, and perindopril was added to patients whenever required to achieve BP of 150/80 mm Hg. The primary outcome measure was a composite of fatal and nonfatal stroke. After a mean follow-up of 1.8 years, BP was 15/6.1 mm Hg lower in the active treatment group, in which a reduction of 30% (95% CI, -1 to 51; P=.06) in the incidence of components of the primary outcome measure was observed, with a reduction of 39% in the risk of death due to stroke (P=.05), of 21% in the risk of death due to any cause (P=.02), and of 64% in the risk of heart failure (P<.0001). Similarly, a lower incidence of serious adverse effects was observed in the active treatment group. This study provides the only evidence available that treatment of HT with indapamide as monotherapy or in combination with perindopril in patients aged over 80 years with the aim to achieving a BP of 150/80 mm Hg is beneficial; however, no data are available that confirm the benefit of larger reductions and, therefore, we should not try to reach normal blood pressures in this large group of patients.
Preventing the Deterioration in the Risk Profile: Prevention of Diabetes, Atrial Fibrillation, and Albuminuria
The reduction in overall cardiovascular risk is the main goal of HT treatment. As mentioned earlier, in addition to reducing BP levels, it is necessary to intervene in other risk factors such as lipid and carbohydrate metabolism, prevention and regression of organ damage, and reduction of the risk of complications.
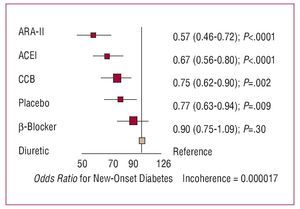
In the last decade, there has been a growing interest in the effect of antihypertensive drugs on the metabolism of carbohydrates and, specifically, the prevention of diabetes. The results of a recent metaanalysis (Figure 10) indicate that, compared with diuretics, calcium channel blockers and, above all, ACE inhibitors and ARA-II, reduce the risk of new-onset diabetes.39 If we take placebo as a neutral effect, the differences only seem to be significant with ACE inhibitors and ARA-II, whereas calcium channel blockers have a neutral effect and b-blockers and diuretics promote the appearance of diabetes.
Figure 10. Results of a metaanalysis of new-onset diabetes in 22 clinical trials that included 143 153 hypertensive patients. ACEI indicates angiotensin converting enzyme inhibitors; ARA-II, angiotensin II antagonists; CCB, calcium channel blockers. Based on Elliot et al.39
Although the investigators of the PIUMA study have reported that hypertensive patients who suffer new-onset diabetes during follow-up have a cardiovascular prognosis as unfavorable as patients with diabetes initially and significantly worse than the group who remain free of the disease, the prognostic significance of this event has yet to conclusively determined.40 Data from a recent publication bring into question the persistence of the antidiabetic effect, which is probably lost in the medium-term.41
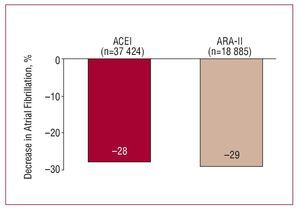
The decrease in BP levels and effects of antihypertensive agents other than BP reduction seem to condition their capacity to prevent the development of atrial fibrillation in hypertensive patients. The results of a recent metaanalysis indicate that drugs that block the renin-angiotensinaldosterone system (ACE inhibitors and ARA-II) have a similar or greater capacity than other groups for reducing the risk of arrhythmia, a capacity which may in part be due to the relationship between angiotensin II and atrial fibrillation42 (Figure 11). Although we only have available the analysis of the subgroup of patients in the LIFE study who developed atrial fibrillation during follow-up, the data indicate that the prevention of arrhythmia might be accompanied by a significantly lower cardiovascular risk, in particular, of stroke and heart failure.43
Figure 11. Primary and secondary prevention of atrial fibrillation with antihypertensive drugs. ACEI indicates angiotensin converting enzyme inhibitors; ARA-II, angiotensin II antagonists. Based on Healey et al.42
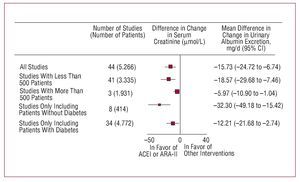
Nephroprotection is another of the aims of antihypertensive treatment. This concept covers avoiding deterioration in renal function, assessed usually by glomerular filtration, and preventing, eliminating, or reducing urinary excretion of albumin. In addition to being indicators of renal function, both these markers of organ damage in hypertensive patients have been shown to bear a close relationship with cardiovascular risk, and in the case of albuminuria, reduction through antihypertensive treatment has been shown to be associated with better cardiovascular and renal prognosis. As in the previous section, the reduction in BP and effects of the drugs other than decreases in BP have been shown to be associated with nephroprotection and, likewise, the compounds that interfere with the activation of the reninangiotensin-aldosterone system have been shown to have a greater capacity than other drugs for preserving glomerular filtration and a greater antiproteinuric efficacy44
Figure 12. Effect of angiotensin converting enzyme inhibitors (ACEI) and angiotensin receptor II antagonists (ARA-II) on urinary excretion of albumin compared to other interventions. Based on Casas et al.44
PHARMACOLOGICAL TREATMENT OF DYSLIPIDEMIA
In this section, we will analyze the current recommendations for treating dyslipidemia, although we shall avoid giving a detailed description of managing patients with genetic dyslipidemias or uncommon clinical presentations of lipid metabolism disorders. There is no doubt about the relationship between high plasma cholesterol and the risk of atherothrombotic cardiovascular complications and the evidence for the relationship between reducing cholesterol and lowering risk is also beyond question. As is the case with HT, the largest benefits of an intervention are seen in highest-risk patients. A reduction of 10% in cholesterol levels is accompanied by a decrease in the 5-year risk of ischemic heart disease of 25%; a reduction of 40 mg/dL in LDL cholesterol (LDL-C) is associated with a 20% decrease in the risk of coronary complications. These decreases in risk depend on the initial risk and the age of the patients.45
Recommendations of the Clinical Practice Guidelines
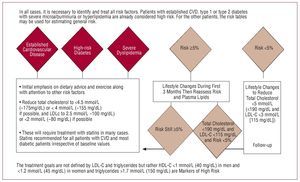
Figure 13 is a schematic diagram of the current recommendations of the new European guidelines for prevention.46 In the general population, it is recommended to aim for total cholesterol levels below 190 mg/dL and LDL-C greater than 115 mg/dL, with stricter lipid goals set according to higher risk. These recommendations are stricter than those proposed by the American guidelines, which also set goals according to risk but with slightly higher values, at least in patients without diabetes and in those with no history of vascular disease.47
Figure 13. Recommendations of the European Guidelines for Cardiovascular Prevention concerning lipid-lowering therapy. Based on Graham et al.46
Although the guidelines do not set any specific goals for HDL-C or triglycerides, HDL-C below 40 mg/dL in men and below 45 mg/dL in women, as well as triglycerides greater than 150 mg/dL are considered as markers of higher cardiovascular risk. Patients with familial hypercholesterolemia with total cholesterol levels above 380 mg/dL and LDL-C above 240 mg/dL are included directly in the high-risk group irrespective of other factors. In particular, those with dyslipidemia since childhood are considered high risk and pharmacological treatment is indicated even in asymptomatic patients.
We currently have a limited therapeutic arsenal for treating dyslipidemias. In addition, the recent results suggest that drugs (torcetrapib) that increase the plasma concentration of high-density lipoprotein cholesterol (HDL-C) through interference with the enzymatic degradation process are not associated with clinical benefit. Specifically, in the only large clinical trial available that compared the drug with placebo, an increase of 25% was seen in the risk of cardiovascular complications.48 In clinical practice, we have at our disposal inhibitors of hydroxy-3-methylglutaryl-CoA-reductase (statins), fibrates, bile acid sequestering resins, nicotinic acid, and selective inhibitors of cholesterol absorption (ezetimibe). Treatment with statins, which are the current treatment of choice, has been shown not only to improve lipid profile, specifically by reducing the plasma concentration of LDL-C, but also to have a favorable effect on cardiovascular prognosis, in particular for coronary outcomes in treated patients (Figures 14 and 15, Table 1).45,49-50 At high doses, it is possible to achieve regression of coronary atherothrombosis with good tolerance. The most common adverse effects are muscular ones, although rhabdomyolysis is uncommon; serious alterations in liver enzyme tests are uncommon and reversible after withdrawal of the medication. In clinical practice, it is necessary to bear in mind possible interactions with other commonly used drugs such as digoxin, warfarin, calcium channel blockers, sildenafil, cyclosporine, etc.46
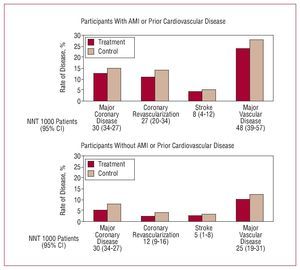
Figure 14. Metaanalysis of data from 90 056 participants in 14 randomized clinical trials with statins. AMI indicates acute myocardial infarction; NNT, number needed to treat. Based on CTT Collaborators.45
Figure 15. Relative risk of serious coronary complications in clinical trials of primary prevention with statins. The combined relative risk was 0.71 (95% CI, 0.60-0.83; P<.001). Based on Thavendiranathan et al.50
Direct inhibitors of absorption can be combined with statins to achieve lipid goals, although a recent publication has called into question their efficacy in carotid remodeling in hypercholesterolemic patients.51 Ongoing clinical trials will help to clarify their role in the treatment of dyslipidemia and cardiovascular prognosis. Resins are effective at reducing LDL-C, although they increase triglyceride levels and there are problems of gastrointestinal tolerance. Nicotinic acid (pending marketing as combination with a compound that limits its adverse cutaneous effects) and fibrates increase HDL-C and reduce triglycerides, and omega fatty acids can be used in patients with hypertriglyceridemia. The combination of statins and fibrates could be useful in certain patients, although their use should be monitored carefully for possible adverse effects.
Clinical Benefits and Prognosis for Treatment of Dyslipidemia: Beyond LDL-C Reduction?
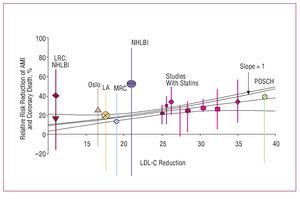
Several metaanalyses published in recent years have analyzed the possibility of benefits beyond those of improved lipid profile. Many experimental and clinical studies have shown that, in addition to the lipid-lowering effect, treatment with statins is accompanied by changes in different markers of cardiovascular risk, in particular, anti-inflammatory and immunomodulatory effects, antiplatelet effects, improvement in endothelial function, prevention of ischemic myocardial, and cerebral damage, etc. In many cases, these changes are closely related to decreases in LDL-C, although some studies have reported truly pleiotropic effects unrelated to changes in the lipid profile. The results of a metaregression analysis indicate that, irrespective of the lipid-lowering strategy, the prognostic benefit of lipid-lowering treatment observed in 125 primary and secondary prevention clinical trials can be explained almost exclusively by decreased LDL-C52 (Figure 16). Therefore, the nonlipid-lowering effects of statins do not seem to make additional contributions to the reduction of cardiovascular risk beyond that derived from reducing LDL-C in both primary and secondary prevention. Therapeutic interventions that do not involve statins (diet, bile acid sequestering agents, and ileal surgery) seem to yield a similar reduction in risk of ischemic heart disease. Therefore, in view of the results of this analysis, rather than possible pleiotropic effects, the choice of statin should be guided by its efficacy at reducing LDL-C according to the lipid goals of each patient. Factors such as cost, drug interactions, and safety are other elements that should be taken into consideration.
Figure 16. Studies of risk of myocardial infarction and LDL-C reduction with diet, resins, surgery, and statins. Based on Robinson et al.52
Pharmacological Treatment of Dyslipidemia in Primary Prevention
Most of the information available in this area comes from studies with statins although, as mentioned, the decision to start pharmacological treatment should be based on the recommendations in the clinical practice guidelines, which establish both an initial threshold for treatment as well as its goals.
The results with other lipid-lowering agents are less relevant although those of the Helsinki Heart Study53 are worthy of mention. That study included men with high lipid levels whose treatment with gemfibrozil was accompanied by a 34% reduction in the risk of coronary death and nonfatal myocardial infarction. In a subgroup analysis, the benefit was concentrated in patients with triglycerides above 200 mg/dL and HDL-C below 42 mg/dL, in whom the decrease in risk was 66%.
The results of the FIELD study54 extend that observation to patients with type 2 diabetes mellitus and no history of cardiovascular disease who were treated with phenofibrate, corresponding to 90% of the 9795 patients enrolled. The 11% reduction in relative risk observed for one of the components of the primary outcome measure was not statistically significant, although a significant difference was found for the secondary outcome measure (myocardial infarction, stroke, cardiovascular death, and need for revascularization) on analyzing the subgroup with no prior cardiovascular disease (19% reduction; P<.004). Once again, benefit appeared to be concentrated in patients with low HDL-C and high triglyceride levels. Without doubt, the results of these studies limit the role of fibrates in current clinical practice guidelines to treatment of dyslipidemia.
Special mention should be made of the results of a study published recently that analyzed the efficacy of high-dose statin therapy compared to combination with a cholesterol absorption inhibitor (ezetimibe) in patients with familial hypercholesterolemia.51 The primary outcome measure of the study was changes in carotid intimal-medial thickness measured by ultrasonography. After 2 years of follow-up, even though a difference of approximately 50 mg/dL in LDL-C was observed in favor of the group assigned to combination therapy, and there was a significant reduction in the plasma concentration of C-reactive protein, no differences in outcome were observed. In spite of these results and while awaiting the conclusions of ongoing prognostic studies, we believe that ezetimibe should continue to be considered as a possible therapeutic alternative in combination with statins. The selection of patients, who for the most part were pretreated with statins, the limited increase in baseline intimal-medial thickness, and the vascular territory studied could have influenced the results.
With statins, the results of a metaanalysis indicate that the reduction in the incidence of serious coronary complications, coronary revascularization procedures, and stroke is proportional to the absolute reduction in LDL-C and that the size of the relative risk reduction is independent of the baseline concentrations of cholesterol and other characteristics (age, sex, or cardiovascular disease).45 However, at least in primary prevention strategies the decision to start treatment should be based, as the guidelines indicate, on the baseline cardiovascular risk of the patients.
In this recommendation, the results of a third metaanalysis of 7 clinical trials that included 42 848 patients, 90% of whom had no history of cardiovascular disease, should also be considered. After a mean follow-up of 4.3 years, the treatment reduced the relative risk of serious coronary events, stroke, and the need for revascularization by 29% (P<.001), 14.4% (P=.02), and ~33.8% (P<.001), respectively. Statin therapy was accompanied by a 22.6% reduction in coronary mortality, a difference that was not statistically significant. Likewise, the difference in all-cause mortality was not statistically significant. It should be highlighted that the benefits are observed regardless of the baseline LDL-C levels and the presence of concomitant risk factors, although a direct association has been described between the size of the benefit, baseline cardiovascular risk, and the size of the decrease in LDL-C. Table 2 shows a comparison of the risk reduction in patients in primary and secondary prevention. Treatment with statins seems to be cost-effective in the primary prevention of patients at high cardiovascular risk with an absolute 10-year risk of coronary artery disease of more than 20% according to the Framingham tables (>5% using the SCORE tables), with an unfavorable cost-effectiveness in those at low risk (<10%). For treatment of patients with intermediate risk, the current clinical guidelines, which limit their systematic use, should be followed. The current recommendations for reducing cardiovascular risk in diabetic patients require, in practice, the use of statins in a high percentage of patients, and the results of the CARDS55 and HPS56 studies have been decisive in the formulation of those recommendations. However, it is possible that young diabetic patients who are free of other risk factors or comorbidities are not a high-risk population, and the therapeutic decision should be based on general recommendations for primary prevention according to the estimation of cardiovascular risk.
In this group of patients, no significant increase in the risk of cancer or hepatic or muscular toxicity was observed, confirming the excellent safety profile of the statins.
CONCLUSIONS
Primary pharmacological prevention of HT and dyslipidemia should be accompanied by lifestyle changes and integrated into a set of therapeutic initiatives aimed at reducing general cardiovascular risk in the population. The interventions, in line with the current recommendations in the clinical practice guidelines, should be adjusted to the baseline risk of the patients with different treatment goals according to that risk. A number of studies have shown the mutual enhancement of efficacy when HT and dyslipidemia are treated pharmacologically. Recent data analyzing changes in markers of subclinical organ damage (left ventricular hypertrophy and carotid intimal-medial thickness) in diabetic American Indians are a good example of the need for multifactorial intervention to manage risk.57
ABBREVIATIONS
ACE: angiotensin converting enzyme BP: blood pressure
CVD: cardiovascular disease
CVRF: cardiovascular risk factors DBP: diastolic blood pressure
HT: hypertension
SBP: systolic blood pressure
Section Sponsored by Laboratorio Dr Esteve
Correspondence:
Dr. J.R. González-Juanatey.
Travesía de A Choupana, s/n. 15706 Santiago de Compostela. A Coruña. España.
E-mail: jose.ramon.gonzalez.juanatey@sergas.es