Fontan-associated liver disease refers to the disturbance in the liver secondary to hemodynamic changes and systemic venous congestion following Fontan surgery. Although the natural history of this disease has not yet been established, patients with more advanced liver injury develop the complications of portal hypertension, such as ascites, variceal haemorrhage, or encephalopathy. Moreover, patients with Fontan surgery may have an increased risk of hepatocellular carcinoma. Periodic liver monitoring is essential to prevent this disease and provide early treatment of liver complications.
Keywords
Fontan-associated liver disease (FALD) refers to a wide range of structural and functional alterations of the liver caused by hemodynamic changes associated with the Fontan circulation (FC). As in all chronic liver diseases, the individual passes through various stages before reaching the final phase, which is when most of the complications appear, such as hepatocellular carcinoma, variceal bleeding, ascites, and hepatic encephalopathy. Although hepatic impairment eventually develops in all patients with FC, its adequate characterization is essential to allow us to determine the degree of liver damage and thereby establish screening and follow-up programs to prevent and/or treat at an early stage any liver complications that may arise. This review summarizes the current evidence on the pathophysiology of FALD, as well as the methods available for its diagnosis and management. Finally, we propose a follow-up strategy tailored to the peculiarities of these patients.
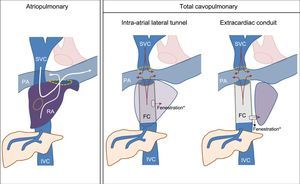
FONTAN SURGERY: DEFINITION, TYPES, AND CONSEQUENCESThe FC is used to treat several complex congenital heart diseases with a common characteristic: the presence of a functional single ventricle. The surgical objective is to ensure that the systemic venous return is directed into the pulmonary artery, bypassing the right ventricle. Simply put, the Fontan technique surgically creates an anastomosis between the systemic venous return from both vena cavae and the pulmonary artery that passively carries the blood to the single ventricular chamber. Therefore, the FC is a curative surgery whose main objective is to mitigate the hypoxemia and prolong survival.1
There are 2 main variations of the technique: the atriopulmonary (classic) procedure and the total cavopulmonary (Figure 1). The classic FC involves transformation of the atrium into a channel connecting both vena cavae to the pulmonary artery. For this, an anastomosis is created between the atrium and the pulmonary artery, and the tricuspid valve and atrial septal defect are closed. Although it was initially thought that maintenance of the participation of the atrium in the circuit would facilitate the propulsion of the blood to the lungs, this approach was later found to increase the risk of atrial tachyarrhythmias and thromboses.2,3 The total cavopulmonary variant, the technique of choice in recent years, is carried out in 2 stages: in the first, the venous return of the superior vena cava is connected to the pulmonary arterial circulation (Glenn procedure), with completion of the procedure at the same time or later with an anastomosis from the inferior vena cava to the pulmonary artery using an artificial conduit (Fontan conduit).
Although the technique was first described in 1968, its use was quite limited until the 1980s.4 Accordingly, its long-term results are unknown. Nonetheless, the FC represents one of the greatest advances in pediatric cardiology, ensuring survival rates of about 80% at 20 years, which must be considered a huge success given the severity of the cardiac anatomic defects addressed.5,6 However, the long-term hemodynamic changes caused by the surgery are now associated with a considerable number of complications that can affect virtually all organs.7,8
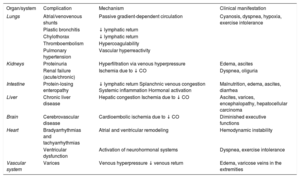
The concept of “Fontan failure” refers to the syndromic set of systemic conditions that develop in the long term in most patients who undergo the procedure. Whatever the cause, the result is always the same: increased systemic venous pressure. Fontan failure can be precipitated by different events, such as heart arrhythmia, stenosis, or obstruction of the Fontan conduit, or, in its most advanced form, ventricular dysfunction. This pressure elevation triggers 2 key phenomena: a) congestion in the splanchnic venous circulation (which is unable to self-regulate flow), and b) decreased lymphatic return via the thoracic duct. Finally, when Fontan failure is accompanied by a deterioration in single ventricular systolic function, cardiac output decreases, which promotes ischemia in target organs. These and the other mechanisms discussed below determine the damage to the different organs and systems, as summarized in Table 1.9–12
Systemic Implications of “Failure” of the Fontan Circulation
| Organ/system | Complication | Mechanism | Clinical manifestation |
|---|---|---|---|
| Lungs | Atrial/venovenous shunts | Passive gradient-dependent circulation | Cyanosis, dyspnea, hypoxia, exercise intolerance |
| Plastic bronchitis | ↓ lymphatic return | ||
| Chylothorax | ↓ lymphatic return | ||
| Thromboembolism | Hypercoagulability | ||
| Pulmonary hypertension | Vascular hyperreactivity | ||
| Kidneys | Proteinuria | Hyperfiltration via venous hyperpressure | Edema, ascites |
| Renal failure (acute/chronic) | Ischemia due to ↓ CO | Dyspnea, oliguria | |
| Intestine | Protein-losing enteropathy | ↓ lymphatic return Splanchnic venous congestion Systemic inflammation Hormonal activation | Malnutrition, edema, ascites, diarrhea |
| Liver | Chronic liver disease | Hepatic congestion Ischemia due to ↓ CO | Ascites, varices, encephalopathy, hepatocellular carcinoma |
| Brain | Cerebrovascular disease | Cardioembolic ischemia due to ↓ CO | Diminished executive functions |
| Heart | Bradyarrhythmias and tachyarrhythmias | Atrial and ventricular remodeling | Hemodynamic instability |
| Ventricular dysfunction | Activation of neurohormonal systems | Dyspnea, exercise intolerance | |
| Vascular system | Varices | Venous hyperpressure ↓ venous return | Edema, varicose veins in the extremities |
CO, cardiac output.
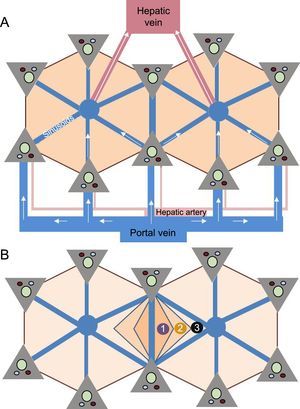
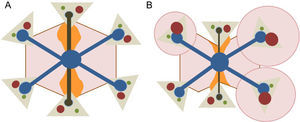
The liver is an organ with a complex dual vascularization architecture that receives two-thirds of its blood through the portal venous system and approximately one-third through the hepatic artery. However, venous drainage only occurs through the hepatic veins, which drain the blood to the inferior vena cava and from there to the heart. This peculiar vascularization explains the microscopic polyhedral lobule architecture of the liver, which is fundamental to the understanding of the pathophysiology of FALD13 (Figure 2).
Microscopic architecture of the liver. A: The main afferent vessels of the liver (portal vein and hepatic artery) are divided into venules and arterioles, which in turn give rise to small vessels that converge in the hepatic sinusoids; the sinusoids are small vascular spaces formed by endothelial cells and surrounded by hepatocytes that enable the distribution of the blood flow and return the blood through the centrilobular veins to the systemic circulation. B: The liver lobule shows a polyhedral architecture whose ends contain the “portal spaces”, formed by small arterial and portal vessels and bile ducts; the centrilobular vein is located in the center of the lobule and is connected to the portal spaces through the sinusoids. Because the portal spaces and the centrilobular vein are anatomically separated, there is an oxygen and nutrient concentration gradient in the blood that is higher in areas close to the portal spaces and lower in those near the centrilobular vein. This gradient allows the lobule to be divided into 3 zones: zone 1 is the periportal region and zone 3 is the pericentral region.
In most liver diseases, the damage is caused through inflammatory mechanisms by the arrival to the liver of toxic products such as alcohol or drugs, viral infections, and other agents. Because the inflammation requires a large amount of oxygen, the damage initially appears in the most oxygenated regions, namely, the periportal zones.14 However, in patients with FC, the pathophysiology is quite distinct because there are no inflammatory phenomena, and the injury model is primarily due to the persistent hepatic congestion.15
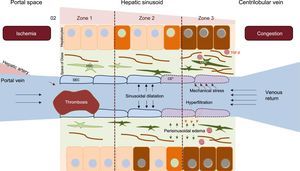
Because of the location of the liver, an elevated central venous pressure is easily transmitted to the liver. If this pressure is maintained, it causes congestion and increased intrahepatic pressure. The result of these phenomena at the microscopic level is sinusoidal dilatation, which is more evident in the centrilobular area. The sinusoidal hyperpressure transmitted from the systemic circulation promotes hyperfiltration of the blood and causes edema to develop in the space between the sinusoidal endothelial cells and the hepatocytes (space of Disse). This edema, coupled with the low cardiac output, limits the arrival of oxygen and facilitates hepatocyte necrosis phenomena and the emergence of profibrogenic mediators such as tumor growth factor beta.16 In addition, exposure of the sinusoidal endothelial cells to the vascular tension and stress induces a phenotypic change that converts them into fenestrated cells that release various mediators. These agents permit activation of the hepatic stellate cells located in the space of Disse that have a large fibrogenic capacity (Figure 3). Although the changes are initially perisinusoidal and in zone 3, they can eventually affect the entire liver lobule via the formation of bridging fibrosis and regenerative areas, which are indicative of cirrhosis (Figure 4).
Pathophysiology of Fontan-associated liver disease. Systemic venous hypertension secondary to the FC decreases venous return, which increases the pressure and dilates the sinusoid in a retrograde manner. Hyperfiltration phenomena occur toward the space of Disse, and mechanical stress induces a phenotypic change in sinusoidal endothelial cells. The release of certain molecules by these cells activates in an autocrine manner hepatic stellate cells, which facilitate fibrogenesis phenomena. Over time, the hypoxia and perisinusoidal fibrosis produce parenchymal hepatocyte necrosis that is more evident in zone 3 (close to the centrilobular vein). HSC, hepatic stellate cell; SEC, sinusoidal endothelial cell.
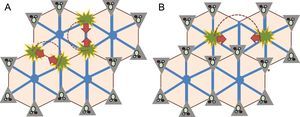
Fibrogenesis in Fontan-associated liver disease. Whereas in the conventional models of cirrhosis (A), the bridging fibrosis appear between the portal spaces, in the model of Fontan-associated liver damage, the bridges are formed between the centrilobular areas (B), which gives rise to the concept of “inverted cirrhosis”, a characteristic of these patients.
Patients with FC show important hemostatic alterations affecting all of the disease stages and mirror those found in patients with advanced liver disease, which confers them with a latent state of hypercoagulability.17 If this state is coupled with intrahepatic endothelial damage and considerable venous stasis, Virchow's triad is perfectly fulfilled, meaning that intrahepatic microthrombotic phenomena that aggravate and perpetuate the liver damage are very likely.18 This hypothesis, although requiring further study, opens the door to alternative therapies, such as anticoagulation, which can reduce the liver damage.
Finally, the liver disease in patients with FC can occur for reasons other than the cardiac surgery itself, such as hepatotoxicity due to amiodarone,19 an antiarrhythmic agent commonly used in this population, or hepatitis C.20
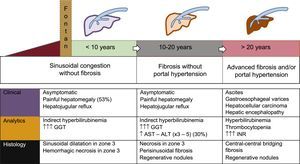
NATURAL HISTORY AND CLINICAL FORMS OF FONTAN-ASSOCIATED LIVER DISEASEFALD is one of the main and often the first manifestation of Fontan failure. Although its natural history remains to be established, it can be generally divided into 3 main stages (Figure 5):
- 1.
Liver congestion and sinusoidal dilatation. This stage begins even before the FC and continues during the first years after the procedure.21,22 About 53% of these patients have painful hepatomegaly and/or hepatojugular reflux, although many are asymptomatic. On blood analysis, this stage is characterized by mild indirect hyperbilirubinemia and a gamma-glutamyl transpeptidase increase that is related to the canalicular congestion secondary to perisinusoidal edema. On biopsy, sinusoidal dilatation and hepatocellular necrosis are evident in zone 3 of the lobule.23
- 2.
Fibrosis without portal hypertension. After between 5 and 10 years, there are signs of perisinusoidal fibrosis, regenerative nodules, and hepatocellular necrosis. At this stage, necrotic phenomena may be accentuated if there is low cardiac output, so there are often slight increases in aspartate aminotransferase, alanine transaminase, and lactate dehydrogenase. The fibrosis is potentially reversible if the patient undergoes heart transplantation.24
- 3.
Advanced fibrosis with portal hypertension. This is the final stage of all liver diseases. It shows hypoalbuminemia, prolonged coagulation times, and decreased platelet counts. At this stage, the risk of hepatocellular carcinoma is increased, as well as portal hypertension complications, such as ascites or bleeding due to rupture of gastroesophageal varices.25
Natural history of Fontan-associated liver disease. The temporal sequence is illustrative because the timeline depends on the course of the heart disease. Arrhythmias or ventricular dysfunction can accelerate the clinical course. ALT, alanine transaminase; AST, aspartate aminotransferase; GGT, gamma-glutamyl transpeptidase.
A distinct clinical entity can be encountered in any of the stages described: ischemic hepatitis, which is characterized by a marked elevation in aspartate aminotransferase, alanine transaminase, and lactate dehydrogenase and takes place in the acute context of low cardiac output. It is usually reversible after resolution of the event underlying the precarious hemodynamic situation.
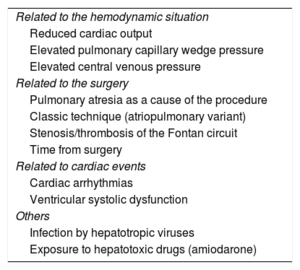
The time course of FALD is difficult to establish because, given that it is not a primary disease of the liver, its clinical course depends on the cardiac situation. The variables associated with increased risk of liver damage are listed in Table 2.26–28 However, the main determinant of liver complications is time, with a risk of decompensation 4 and 9 times higher 15 and 20 years after FC than after 5 years, respectively.29
Risk Factors for Liver Damage in Patients With the Fontan Circulation
| Related to the hemodynamic situation |
| Reduced cardiac output |
| Elevated pulmonary capillary wedge pressure |
| Elevated central venous pressure |
| Related to the surgery |
| Pulmonary atresia as a cause of the procedure |
| Classic technique (atriopulmonary variant) |
| Stenosis/thrombosis of the Fontan circuit |
| Time from surgery |
| Related to cardiac events |
| Cardiac arrhythmias |
| Ventricular systolic dysfunction |
| Others |
| Infection by hepatotropic viruses |
| Exposure to hepatotoxic drugs (amiodarone) |
The liver damage in patients with FC is universal. However, because not all patients manifest the complications, methods are required to permit the identification of at-risk individuals who would benefit from close and directed follow-up.30 For compensated patients, the only diagnostic method currently allowing an adequate staging of liver disease is biopsy. However, recent advances in hepatology have attempted to develop noninvasive diagnosis methods, which have also been evaluated in patients with FC.
Serological MethodsA complete blood count may be sufficient as a diagnostic strategy for liver disease in patients with FC. A platelet count less than 150 000/μL is the main indicator of hypersplenism, one of the clearest analytical findings of portal hypertension. Multiple simple methods have been developed in recent years that correlate various analytical parameters with each other and with certain radiological findings. Although these methods were developed to diagnose liver cirrhosis with other indications besides FC, many have been used for this indication (Table 3). The main drawback is that they have not yet been validated against the reference standard, liver biopsy, which is why no validated cutoff points are available. Nonetheless, several methods were comparatively evaluated in a cohort of 204 patients29; 26% had signs of hepatic decompensation. The Forns index was determined to be the best predictor of the presence of advanced liver damage, with an area under the curve of 0.786. In patients with advanced liver damage of any cause, the MELD (Model for End-stage Liver Disease) scale is a very useful prognostic tool, so much so that it is used in most centers to establish the indication and priority for liver transplantation. Its main disadvantage is that it relies on the INR, which is artificially elevated by some anticoagulants commonly used in patients with FC. However, the MELD-XI scale does not include the INR and has been created for patients treated with oral anticoagulants.31 Although the MELD-XI has been correlated with the degree of fibrosis, it has not been possible to define an appropriate cutoff point to categorize the FALD.32
Serological Diagnostic Methods for Patients With the Fontan Circulation
| Marker | Parameters included | Evidence |
|---|---|---|
| APRI | AST, platelets | Demonstrated correlation with radiological findings |
| AST/ALT | AST, ALT | Demonstrated correlation with radiological findings |
| FIB-4 | Age, AST, ALT, platelets | Demonstrated correlation with radiological findings |
| Forns index | Age, AST, cholesterol, platelets | Demonstrated correlation with radiological findings |
| ELF score | TIMP-1, PIIINP, and hyaluronic acid | Has not been shown to be useful |
| Platelets | Total platelet count | Demonstrated correlation with radiological findings |
| MELD-XI | Bilirubin, creatinine | Demonstrated correlation with radiological findings. Without cutoff points with adequate sensitivity and specificity |
| FibroSure | ALT, A2-macroglobulin, apolipoprotein A-1, bilirubin, GGT, haptoglobin, AST, glucose, total cholesterol, and triglycerides | Correlation with histologic findings not shown |
ALT, alanine transaminase; APRI, AST to platelet ratio index; AST, aspartate aminotransferase; ELF, enhanced liver fibrosis; FIB-4, fibrosis-4; GGT, gamma-glutamyl transpeptidase; MELD-XI, Model for End-stage Liver Disease-XI; PIIINP, N-terminal polypeptide of collagen type III; TIMP-1, tissue inhibitors of metalloproteinases-1.
This is the method of choice in the initial evaluation of all patients with suspected chronic liver disease and typically enables its diagnosis thanks to the signs listed in Table 4. An irregular outline of the parenchyma on a high-frequency transducer is the most specific ultrasound finding for the diagnosis of advanced liver disease.33–35 Additionally, portal flow inversion is 100% specific for the diagnosis of portal hypertension.36 A combination of analytical and radiological methods, such as the platelet count/spleen diameter ratio, can facilitate the diagnosis of portal hypertension and select patients at risk of the development of gastroesophageal varices.37 In patients with FALD, the most frequent echographic findings are heterogeneous echogenicity, a nodular surface, and small hyperechoic nodules.35,38 Kutty et al.,39 via a controlled Doppler study of 106 individuals, showed higher resistance and pulsatility indexes in the celiac artery and superior mesenteric artery, with a significant reduction in portal velocity in patients with FC. The loss of the triphasic Doppler pattern of the hepatic veins is universal in the total cavopulmonary variant (due to absence of atrial beat transmission), but the presence of a monophasic pattern indicates advanced liver damage.26
Ultrasound Findings Indicating Advanced Liver Damage and Portal Hypertension in Fontan-associated Liver Disease
| B-mode |
| Dull or nodular liver edges |
| Heterogeneity of the hepatic parenchyma |
| Intrahepatic venovenous shunts |
| Portal vein diameter > 12mm |
| Splenomegaly (area > 50cm2) |
| Doppler |
| Decreased portal velocity (< 16cm/s) |
| Portal flow inversion |
| Hepatic arterial resistive index > 0.71 and pulsatility index > 1.3 in the hepatic artery |
| Monophasic or biphasic flow in the suprahepatic veins |
This simple noninvasive diagnostic method permits the measurement of liver stiffness. A transducer emits a mechanical vibration in the form of a low-frequency and high-amplitude sound waves that reach the liver tissue and generate a longitudinal wave that is registered by the transducer. The more rigid the tissue, that is, the more fibrosis it has, the higher the transmission speed of the wave. The speed is converted by mathematical algorithms into stiffness, measured in kilopascals.40 There are 2 main elastographic methods: transient elastography (FibroScan), which is simpler and more widely used in Europe, and sonoelastography, which requires an experienced operator and is more popular in North America. Elastography allows classification of patients in the 4 classic stages of fibrosis and has been validated for practically all etiologies, eliminating the need for many of the previously performed biopsies.30 One of its main drawbacks is the false positives that can occur in a number of situations, such as liver congestion.41 This means that elastography values can be overstated in patients with FC. In fact, the procedure induces an almost immediate elevation in elastography values that is solely due to the liver congestion.42 Nonetheless, as the years pass and signs of Fontan failure and liver damage appear, the transient elastography values eventually exceed 15kPa, probably due to the fibrosis.43,44 A proof-of-concept study performed using sonoelastography with the shear-wave technique showed a positive correlation between histological liver damage and rigidity.39 Therefore, although the appropriate cutoff points for the diagnosis of advanced liver disease are unknown, elastography can be a useful tool.
Magnetic Resonance ImagingMagnetic resonance imaging can be very useful in FALD.45 Its main advantage in this scenario is its ability to diagnose and characterize hepatic nodules. In addition, because cardiac magnetic resonance is currently one of the main tools used during follow-up in this population, it can be combined with dynamic hepatic magnetic resonance to minimize patient discomfort and optimize resources. Although its use is not yet widespread due to its high cost, magnetic resonance elastography can also evaluate fibrosis; indeed, there is a positive correlation between the stiffness values obtained with this method and the APRI index, MELD score, Fontan circuit pressure, and even histological damage.46
Hepatic HemodynamicsMeasurement of the hepatic venous pressure gradient (HVPG) may help to better grade the FALD and can even be a tool to aid in the differential diagnosis of ascites. The procedure is quick, simple, and minimally invasive and does not require sedation.47 The HVPG is defined as the difference between the wedge and free pressures in one of the hepatic veins, preferably the right. Measurement of the HVPG permits the differential diagnosis of portal hypertension symptoms, with some elevated pressures with normal HVPG indicating a posthepatic origin, which is the most common post-FC finding.27 In the presence of advanced parenchymal damage, the HVPG can exceed 6mm Hg, even 10mmHg, a threshold that is considered to indicate risk of decompensation in most liver diseases.48 However, various circumstances can lead to underestimation of the HVPG, such as the presence of vascular fistulas between the hepatic veins themselves or between these veins and the portal branches. In our experience, such fistulas are very frequent in patients with FC, which complicates the interpretation of the HVPG result and means that experienced staff is required to perform the measurement.
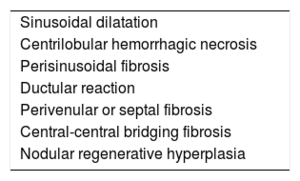
Liver BiopsyThis is the diagnostic reference standard and, because the noninvasive diagnosis methods remain to be adequately validated, is still necessary to irrefutably determine the liver damage. The biopsy can be performed via a transjugular approach, together with the HVPG measurement, or via percutaneous access. The characteristic histologic findings are reported in Table 5. Sinusoidal dilatation is present in 90% to 97% of patients with FC and is the earliest finding. Characteristically, the sinusoidal dilatation is more pronounced than for other causes of cardiogenic cirrhosis. The fibrosis distribution in early stages is typically perisinusoidal (in the space of Disse), an indicator that is absent from other liver diseases of cardiac origin. The most severe and delayed finding is that of extensive centrilobular bridging fibrosis with regenerative nodules, which are usually indicative of an irreversible disease state. Periportal inflammation is generally absent, allowing its differential diagnosis with other liver disease etiologies.49 Some authors recommend a biopsy in all patients 10 years after the FC.25 In a cohort of 67 patients, this strategy revealed that 100% of them had developed liver fibrosis and that its degree increased with time. However, because it has not been possible to correlate the degree of fibrosis with clinically relevant events or hemodynamic or analytical parameters,50 its usefulness as a prognostic marker that facilitates decision-making is still unclear. Currently, a liver biopsy is recommended in patients with liver disease of unknown etiology and in heart and/or liver transplantation candidates.39,40
LIVER COMPLICATIONS OF THE FONTAN SURGERYAdvanced stages of FALD, similar to other forms of liver cirrhosis, may show gastroesophageal varices, ascites, hepatic encephalopathy, hepatocellular carcinoma, and splenomegaly with thrombocytopenia. No study has evaluated in detail the characteristics or natural history of these complications in FALD.
Hepatic Nodules and Hepatocellular CarcinomaLarge regenerative nodules are frequently found in the liver parenchyma in FALD, as in other congestive liver diseases.15,51 These nodules are usually multiple, hypervascular in the arterial phase and hyperechoic in the ultrasound, and less than 3cm in size, located in the liver periphery, and show a prevalence that is directly proportional to the time since the surgery. In the histological study, these nodules are typically regenerative, adenomas, or of focal nodular hyperplasia.28,38,52,53 Although the pathophysiology of benign liver nodules in patients with FALD is unknown, their peripheral location and radiological behavior indicate a vascular origin (Figure 6).54
Nodular regenerative hyperplasia in Fontan-associated liver disease. A: Liver congestion and decreased cardiac output decrease the portal blood flow in the more peripheral regions of the liver and cause focal ischemia. B: Extinction of the parenchyma secondary to ischemia triggers an arterial vasodilatory response that stimulates the proliferation of healthy hepatocytes in the form of nodular regenerative hyperplasia.
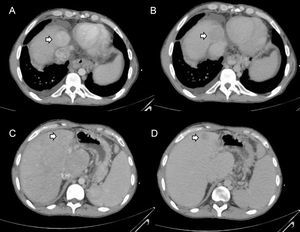
In recent years, isolated cases or small series have been published on hepatocellular carcinoma in patients with FALD.54–67 These patients usually have advanced liver disease and a time from surgery greater than 5 to 10 years. Malignant nodules tend to be hypervascular in the arterial phase, with clearance in the portal phase, and show increased alpha-fetoprotein (Figure 7). However, these characteristics are not pathognomonic and may be absent in some patients. In addition, their diagnostic accuracy in FALD remains to be evaluated. Thus, the differential diagnosis of nodules in FALD remains to be defined. Additionally, the criteria of hepatocellular carcinoma used in cirrhosis have not been validated in other liver diseases such as FALD. Thus, the diagnosis of hepatocellular carcinoma in patients with FALD always requires histological confirmation.46
Radiological diagnosis of hepatocellular carcinoma. These 4 images are from the same patient with Fontan surgery who had 2 focal lesions; biopsies were taken. A: Hepatocellular carcinoma showing hyperdensity in the arterial phase. B: Hepatocellular carcinoma showing hypodensity in the delayed phase. C: Benign peripheral nodule without arterial hyperdensity. D: Benign peripheral nodule with clearance in the venous phase.
Screening every 4 to 12 months using imaging techniques is cost-effective and improves the survival of patients with cirrhosis because it allows early detection of hepatocellular carcinoma and offers curative treatments.68 Nandwana et al.69 retrospectively analyzed a cohort of 145 patients with FC who periodically underwent a liver imaging test; the authors found 1 case of hepatocellular carcinoma in the first imaging test and 4 incident cases after a median follow-up of 3.05 years. Most experts recommend periodic screening, although the optimal interval and imaging tests are unknown.25,52 Hepatocellular carcinoma management should adhere to the clinical practice guidelines for other forms of cirrhosis.68
Gastroesophageal VaricesThe prevalence of gastroesophageal varices is estimated to range between 2% and 43% after a FC.25,30,70 In a retrospective study of 73 patients, the concomitance of varices with other manifestations of portal hypertension was associated with an increased risk of death, heart transplantation, and hepatocellular carcinoma.70 Because cases have been published of gastroesophageal variceal bleeding, some with a fatal outcome, screening and proper prevention should be systematic.25 The prophylactic mainstay of variceal bleeding in other forms of cirrhosis is the use of noncardioselective beta-blockers; however, their effects have not been analyzed in this patient group.71 Because the portal hypertension model in patients with FALD is characteristically hypodynamic, in contrast to the other forms of cirrhosis, the efficacy of these drugs is questionable. In addition to the possible deleterious effects of beta-blockers on the FC itself, elastic band ligation is proposed as a method of primary and secondary prophylaxis. An acute episode of gastrointestinal bleeding due to variceal rupture should be treated with vasoactive drugs (somatostatin, terlipressin, or octreotide) and endoscopic therapy (band ligation). In cases of bleeding refractory to standard treatment, the creation of an intrahepatic portosystemic shunt (IPSS) improves survival in other types of liver disease71; however, the excessive flow of blood to the FC from the splanchnic territory can precipitate a clinical profile of pulmonary hypertension and heart failure. The bibliography contains only 1 case in which IPSS placement controlled variceal bleeding; however, IPSS use should be restricted to highly selected nonresponders to conventional treatment that maintain good heart function.72
AscitesAscites is a late manifestation of liver cirrhosis that is associated with worse survival and quality of life.73 It is the most frequent clinical liver decompensation, with a prevalence ranging between 2% and 17% in patients with FC.52 In chronic liver disease with intrahepatic portal hypertension, ascites appear when the HVPG is ≥ 10mmHg, a level that also has prognostic value.48 However, in FALD, the HVPG is usually normal and ascites can occur in the absence of liver cirrhosis, which is why its value as a prognostic marker and its physiopathological mechanisms do not overlap those of other liver diseases.28,74 Because there are several causes of ascites in patients with FC (Table 6), appropriate differential diagnosis is essential.74 Regardless, to a greater or lesser extent, it is always accompanied by liver damage and is its most evident clinical marker.
Causes of Ascites in Patients With the Fontan Circulation
| Systemic venous hypertension due to Fontan circulation failure (arrhythmias, conduit thrombosis/stenosis, ventricular dysfunction, pulmonary hypertension) |
| Portal hypertension of posthepatic origin |
| Sinusoidal portal hypertension (advanced liver fibrosis) |
| Hypoalbuminemia/hypoproteinemia secondary to protein-losing enteropathy |
Ascites is usually treatable by optimizing cardiac function and nutrition and by using loop diuretics and aldosterone antagonists because the renin-angiotensin-aldosterone system is increased in patients with portal hypertension and heart failure.48,75 Repeated paracentesis is a rescue option, although no FALD series have shown it to be necessary.
Hepatic EncephalopathyHepatic encephalopathy is defined as a brain dysfunction secondary to liver failure and/or portosystemic shunts. It manifests as a broad spectrum of neurological and psychiatric disorders ranging from subclinical changes to coma. An estimated 30% to 40% of patients with cirrhosis will show this clinical profile at some point in clinical course,76 although it is a poorly documented event in FALD. Only 3 cases have been published25,30,77; nonetheless, its incidence and prevalence are probably underestimated by the retrospective nature of the work and by the low possibility that hepatic encephalopathy is considered in the differential diagnosis.
Liver TransplantationLiver transplantation is the treatment of choice for decompensated cirrhosis with a MELD score > 15 and in certain patients with hepatocellular carcinoma. In the 20th century, cirrhosis was considered an irreversible illness; however, numerous studies of viral and alcoholic cirrhosis show that the fibrosis can be partially or even completely reversed by elimination of the toxic agent causing the liver damage.78,79 Regarding cirrhosis of cardiac origin, experimental models and small case series indicate that the liver disease can also improve and even return to normal if heart function is restored.74,80,81 Because the severity of the heart disease is directly related to the amount of liver damage and that this is universal to a greater or lesser degree, the main unknown is which subgroups of patients require a heart transplantation alone or a double transplantation.
The 2 most significant studies to analyze this issue were retrospective, had a small sample size, and suffered from methodological limitations.82,83 They stressed the good prognosis of heart transplantation in compensated cirrhosis and of double transplantation but did not include patients with decompensated liver disease. Regardless, the results of both studies open an interesting debate on whether double transplantation should be considered for patients with suspected advanced liver disease before the decompensation or whether it should be reserved for those with complications. From our perspective, double transplantation in patients with compensated liver disease is too aggressive, because the severity of the liver damage in these patients is probably insufficient to justify the liver transplantation, particularly given the current shortage of organs, the surgical morbidity involved, and the potential improvement in liver function after heart transplantation alone. In addition, no study has shown that compensated liver disease is a perioperative risk factor or an indicator of poor long-term prognosis after heart transplantation. There is currently no consensus on when to perform a double transplantation. The most experienced institutions in this field recommend an individual analysis of each patient in a multidisciplinary committee.
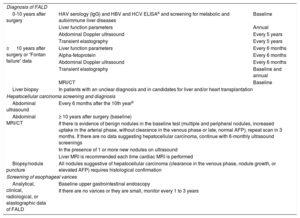
CONCLUSIONSAll patients with FC develop chronic liver disease. Although its natural history is poorly understood and depends on the course of the heart disease, the presence of liver disease should be systematically investigated 10 years after the surgery. In recent years, due to the reported cases of hepatocellular carcinoma, interest has grown in FALD. Its peculiar pathophysiology and clinical behavior make FALD a unique type of liver disease requiring a specific approach that must involve a hepatologist. In our experience, these patients must undergo a periodic liver follow-up (Table 7). Because many questions remain to be answered about the pathophysiology and management of FALD, the creation is required of multicenter research groups that can accumulate sufficient scientific evidence to establish the optimal follow-up of these patients.
Liver Follow-up Recommendations After Fontan Surgery From the Hospital Universitario Ramón y Cajal
| Diagnosis of FALD | ||
| 0-10 years after surgery | HAV serology (IgG) and HBV and HCV ELISAa and screening for metabolic and autoimmune liver diseases | Baseline |
| Liver function parameters | Annual | |
| Abdominal Doppler ultrasound | Every 5 years | |
| Transient elastography | Every 5 years | |
| ≥ 10 years after surgery or “Fontan failure” data | Liver function parameters | Every 6 months |
| Alpha-fetoprotein | Every 6 months | |
| Abdominal Doppler ultrasound | Every 6 months | |
| Transient elastography | Baseline and annual | |
| MRI/CT | Baseline | |
| Liver biopsy | In patients with an unclear diagnosis and in candidates for liver and/or heart transplantation | |
| Hepatocellular carcinoma screening and diagnosis | ||
| Abdominal ultrasound | Every 6 months after the 10th yearb | |
| Abdominal MRI/CT | ≥ 10 years after surgery (baseline) | |
| If there is evidence of benign nodules in the baseline test (multiple and peripheral nodules, increased uptake in the arterial phase, without clearance in the venous phase or late, normal AFP), repeat scan in 3 months. If there are no data suggesting hepatocellular carcinoma, continue with 6-monthly ultrasound screenings | ||
| In the presence of 1 or more new nodules on ultrasound | ||
| Liver MRI is recommended each time cardiac MRI is performed | ||
| Biopsy/nodule puncture | All nodules suggestive of hepatocellular carcinoma (clearance in the venous phase, nodule growth, or elevated AFP) requires histological confirmation | |
| Screening of esophageal varices | ||
| Analytical, clinical, radiological, or elastographic data of FALD | Baseline upper gastrointestinal endoscopy | |
| If there are no varices or they are small, monitor every 1 to 3 years | ||
AFP, alpha-fetoprotein; CT, computed tomography; FALD, Fontan-associated liver disease; HAV, hepatitis A virus; HBcAb, hepatitis B virus core antibody; HBsAb, hepatitis B virus surface antibody; HBsAg, hepatitis B virus surface antigen; HBV, hepatitis B virus; HCV, hepatitis C virus; MRI, magnetic resonance imaging.
Perform HAV serology (IgG) and HCV and HBV ELISA (HBsAg, HBsAb, and HBcAb) in all patients with Fontan surgery. If this patient is not immunized, vaccinate against HAV and HBV and evaluate vaccine effectiveness with new serological tests. After 10 years of effective vaccination against HBV, HBsAb levels must be determined and the vaccination repeated if they are < 100 IU/L.
None declared.
.
We would like to thank the Cardiology Service of the Hospital Universitario Ramón y Cajal for their enthusiastic and unending dedication to FALD.