Catecholaminergic polymorphic ventricular tachycardia (CPVT) is an inherited disease characterized by polymorphic or bidirectional ventricular arrhythmias (VA) triggered by physical or emotional stress in young people with a structurally normal heart. Beta-blockers are the cornerstone of treatment, while flecainide has recently been incorporated into the therapeutic arsenal. The aim of this study was to report our experience with this drug.
MethodsThe cohort included 174 genotype-positive CPVT-patients from 7 families. We collected data from patients who were receiving flecainide and analyzed the indications, adverse effects and dosage, clinical events, VA and arrhythmic window during exercise testing, and implantable cardioverter-defibrillator (ICD) shocks during follow-up.
ResultsEighteen patients (10.4%) received flecainide; 17 patients in combination with beta-blockers, and 1 patient as monotherapy due to beta-blocker intolerance. None of the patients presented side effects. In 13 patients (72.2%) the indication was the persistence of exercise-induced VA and in 5 patients (27.7%) persistent ICD-shocks, despite on beta-blockers. After flecainide initiation, the exercise-induced VA quantitative score was reduced by more than 50% in 66.7% of the members of family 1 (32.76 ± 84.06 vs 74.38 ± 153.86; P = .018). The arrhythmic window was reduced (5.8 ± 11.9 bpm vs 19.69 ± 21.27 bpm; P = .007), and 4 of 5 patients with appropriate ICD shocks experienced no further shocks in the follow-up.
ConclusionsIn CPVT-patients flecainide reduces clinical events, exercise-induced VA, the arrhythmic window, and ICD shocks, with good tolerance.
Keywords
Catecholaminergic polymorphic ventricular tachycardia (CPVT) is a genetic disease characterized by syncope or sudden cardiac death in young people without structural heart disease under physical or emotional stress. Although its prevalence is low, an estimated 1:10 000 in Europe, its lethality is high because sudden death is the first manifestation of the disease in up to 30% of untreated individuals younger than 40 years.1 Mutations thus far described in 6 genes can be used to genetically diagnose 70% of CPVT patients, with 60% of these individuals being carriers of a mutation in the gene encoding the cardiac ryanodine receptor (RyR2).2,3
Catecholaminergic polymorphic ventricular tachycardia patients have a normal resting electrocardiogram and echocardiogram, and diagnosis relies on an exercise test (ET) that triggers increasingly complex ventricular arrhythmias (VAs) as workload increases and/or a genetic study that identifies a pathogenic mutation.4 Given that the ET results are not reproducible, and may even be negative5, genetic screening is essential to diagnose mutation carriers without phenotypic disease expression.4
The fatal prognosis of patients with CPVT has improved since the introduction of beta-blockers, considered the cornerstone of the pharmacological treatment of CPVT.4 Nonselective beta-blockers such as nadolol and propranolol are the most widely recommended drugs because they achieve a greater reduction in VAs and arrhythmic window during ETs.6 However, their protection is incomplete4 and their use is limited by poor tolerance. In addition, during follow-up, up to 30% of patients with CPVT receive an implantable cardioverter defibrillator (ICD)7 but possible complications with these devices and their lack of effectiveness with certain VAs are a problem with this approach.8–12 Accordingly, it is vital to avoid ICD shocks in CPVT patients by using appropriate medication.
The role played by flecainide is increasingly important. This antiarrhythmic agent is now recommended in the latest clinical practice guidelines for patients with recurrent syncope or persistent VA despite beta-blocker therapy.4
The aim of the present study was to analyze the characteristics of genotype-positive CPVT patients under treatment with flecainide and to study its safety and effectiveness.
METHODSIn the present multicenter and bidirectional study, 252 patients with genotype-positive CPVT were identified from 7 families in the Canary Islands. The study involved a prospective intervention in family 1 but was retrospective in the other families. Once participants provided written informed consent, 174 patients with a clinical diagnosis and/or a pathogenic mutation in the RyR2 gene were included; follow-up took place between December 2007 and October 2015.
Genetic AnalysisA Sanger genetic study of the RyR2 and CASQ2 genes was performed in the probands of family 1, followed by massive sequencing of 195 genes to rule out the involvement of other genes. With massive sequencing, 195 genes were also studied in the probands of families 2 and 6, as well as 10 and 8 genes in the index cases of families 4 and 7, respectively. No data were available on the number of genes analyzed in families 3 and 5. Once a mutation was identified in RyR2, cascade screening was performed.
Clinical ProtocolAt diagnosis, all individuals underwent an electrocardiogram, echocardiogram, ET, and genetic study, as well as clinical follow-up and an ET when considered opportune by the treating clinician. Members of family 1 were administered a follow-up and treatment protocol consisting of serial ETs with titration of the beta-blocker dose.13
Analysis was performed of all patients who took flecainide without modification of their baseline dose of beta-blockers. Flecainide was initiated with 2 daily doses until the maximum tolerated dose was reached or a maximum of 200mg/d.
Symptom and Arrhythmic Event DefinitionSymptoms were defined as the occurrence of syncope (nonvasovagal) or dizziness associated with physical or emotional stress. Arrhythmic events were defined as the occurrence of an appropriate ICD shock, syncope, or sudden cardiac death.
Definition and Quantification of Ventricular ArrhythmiasVentricular arrhythmias were defined as any VA, including premature ventricular contractions, bigeminy, couplets, and sustained or nonsustained ventricular tachycardia, and complex VA as any VA except premature ventricular contractions.
To quantify ET-induced VAs, the qualitative scoring system proposed by Van der Werf et al.14 was applied, which uses the highest score obtained in the ET, as well as the quantification scoring system proposed by Wangüemert et al.13, which sums all of the VAs during the ET and scores them according to their severity.
In family 1, the arrhythmic window6 was evaluated, which reflects the range of heart rates (HRs) at which ET-induced VAs occurred; it was measured from the HR at the first VA to the maximum HR.
Exercise tests were performed using a treadmill with the BRUCE protocol. We considered the ET performed before flecainide initiation and the first performed with maximum doses, without modification of the beta-blocker dose. All patients underwent a qualitative analysis of ET-induced VAs; additionally, all of the VAs detected, the time of the tests, the resting and maximum HR, the arrhythmic window, and the quantitative score were also obtained from the patients in family 1.
Statistical AnalysisQualitative variables are reported as absolute values and percentages and quantitative variables as the mean ± standard deviation. To determine the associations among quantitative and qualitative variables, the Wilcoxon and McNemar tests were used, respectively. The Student t test or Mann-Whitney U-test was used for dichotomous qualitative variables and different continuous variables. In repeated measures analysis, due to the small number of observations, the Wilcoxon and McNemar tests were used for quantitative and qualitative variables, respectively. P < .05 was considered statistically significant. Data were analyzed using SPSS statistical software.
RESULTSPatient CharacteristicsAfter a mean follow-up of 5.6 years, 31 of the 174 patients (17.8%) did not receive pharmacological therapy by their own volition, 142 (81.6%) were treated with beta-blockers, and 1 (0.6%) received flecainide monotherapy. At the end of follow-up, 53.4% of patients were under treatment with nonselective beta-blockers, mainly propranolol. Despite beta-blocker therapy, 15% of patients had some type of arrhythmic event, although no patients died during follow-up.
Eighteen patients (10.4%) received flecainide: 17 in combination with beta-blockers and 1 as monotherapy due to intolerance (symptomatic bradycardia) to various beta-blockers.
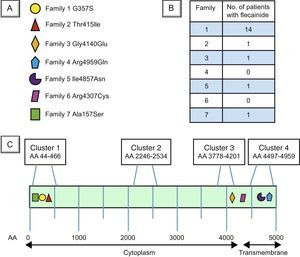
The mean age of patients taking flecainide was 32.3 ± 15.8 years; 50% were men. The mean patient follow-up after flecainide initiation was 2.63 ± 1.28 (range, 0.47-5.75) years. Patients’ baseline characteristics are detailed in Table 1. Mutations diagnosed in each family are shown in Figure 1A, the number of patients under treatment with flecainide and beta-blockers in each family in Figure 1B, and the structure, mutational clusters, and mutations in RyR2 in the families in our cohort in Figure 1C.
Baseline Characteristics of Patients Treated With Flecainide.
| Index cases | 6 (33.3) |
| Genotype-positive relatives | 12 (66.6) |
| Age at diagnosis, y | 28.3 ± 16.1 |
| Sex, male/female | 10/18 (55.5/45.5) |
| Age at flecainide initiation, y | 32.3 ± 15.8 |
| Treated with nonselective beta-blockers (n = 17) | 13 (76) |
| ICD recipients | 14 (77.8) |
| Left cardiac sympathetic denervation | 2 (11) |
ICD, implantable cardioverter defibrillator.
Values represent No. (%) or mean ± standard deviation.
Locations of the RyR2 gene mutations in the 7 families with catecholaminergic polymorphic ventricular tachycardia in the Canary Islands. A: The mutations in each family in our cohort, with the corresponding symbol indicating their location in B. B: Number of patients in each family under treatment with flecainide and beta-blockers. C: Schematic representation of the RyR2 protein. Clusters 1, 2, 3, and 4 represent the protein regions with most mutations described in the literature (hot spots3). AA, amino acid.
All patients are still alive and none had secondary effects obligating therapy discontinuation.
The indication for flecainide addition to beta-blocker therapy was persistence of ET-induced complex VAs in 13 patients (72.2%) and repeat appropriate ICD shocks in 5 (27.8%).
Of those treated with flecainide, 14 patients (77.8%) had an implanted ICD. Eleven patients were prescribed flecainide therapy after ICD implantation and the mean time from ICD implantation to antiarrhythmic agent introduction was 6.1 years.
Two of the patients underwent left cardiac sympathetic denervation, 1 before flecainide initiation.
Flecainide DoseThe mean flecainide dose at the end of follow-up was 159.38mg (2.3mg/k/d). No patient exceeded a dosage of 200mg/d, all had a dosage higher than 100mg/d, and the mean dose in patients with a greater than 50% reduction in the VA quantitative score in family 1 was 123.80 ± 38.31mg.
Clinical ImpactAfter beginning antiarrhythmic therapy, 1 patient (5.5%) remained symptomatic and had a syncopal episode; another patient (5.5%) had an appropriate ICD shock during follow-up.
Impact on Arrhythmia Burden in the Exercise TestIn total, 65 ETs were performed in 17 patients (1 member of family 1 without a postflecainide ET was excluded). No patient had a greater arrhythmia burden in the follow-up ETs, 61% had complete suppression of the arrhythmia burden in the ETs, and only 1 patient (5.5%) had complex VAs in the consecutive follow-up ETs. Of the 13 patients in family 1 whose quantitative scores were compared, 66.7% showed a VA reduction of more than 50% in the ETs (Table 2).
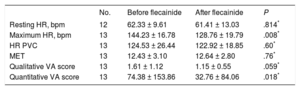
Comparison of Exercise Test Results From Before Flecainide Initiation and With Maximum Doses in Family 1.
| No. | Before flecainide | After flecainide | P | |
|---|---|---|---|---|
| Resting HR, bpm | 12 | 62.33 ± 9.61 | 61.41 ± 13.03 | .814* |
| Maximum HR, bpm | 13 | 144.23 ± 16.78 | 128.76 ± 19.79 | .008* |
| HR PVC | 13 | 124.53 ± 26.44 | 122.92 ± 18.85 | .60* |
| MET | 13 | 12.43 ± 3.10 | 12.64 ± 2.80 | .76* |
| Qualitative VA score | 13 | 1.61 ± 1.12 | 1.15 ± 0.55 | .059* |
| Quantitative VA score | 13 | 74.38 ± 153.86 | 32.76 ± 84.06 | .018* |
HR, heart rate; HR PVC, HR at the occurrence of premature ventricular contractions; VAs, ventricular arrhythmias.
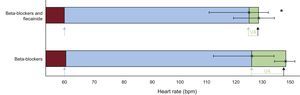
As shown in Figure 2, the arrhythmia window was significantly smaller with both drugs than with beta-blockers alone (5.8 ± 11.9 bpm vs 19.69 ± 21.27 bpm; P = .007) and there were no significant differences in the resting HR—62.3 bpm (95% confidence interval [95%CI], 56.9-67.7) vs 61.4 (95%CI 54.0-68.8)—and the HR at the occurrence of VAs in the ETs—124.5 bpm (95%CI, 110.2-138.9) vs 122.9 (95%CI, 112.7-133.2). Combined flecainide and beta-blockers significantly reduced the maximum HR vs beta-blockers alone—128.76 bpm (95%, 118.0-139.5) vs 144.23 (95%CI, 135.1-153.3) (P < .008)–without a change in workload in the ETs (12.43 vs 12.64 MET; P = .76).
Arrhythmic window in patients during beta-blocker therapy and during treatment with beta-blockers and flecainide. The blue bars indicate the arrhythmia-free HR during ETs, the maroon bars indicate the resting HR, and the green bars indicate the HR at which VAs occurred. The blue arrows indicate the HR at ET initiation, the green arrows indicate the HR at which the VAs start, and the black arrows the maximum HR. The green horizontal lines indicate the arrhythmic window. ET, exercise test; HR, heart rate; VA, ventricular arrhythmia.
Of the 50 ICD recipients in the entire cohort of patients with CPVT, 14 (28%) were treated with flecainide; in 11 of these (22%), the flecainide therapy began after ICD implantation. Flecainide was added to 5 patients due to repeated appropriate ICD shocks; 4 of these patients had no more shocks in a mean follow-up of 2.5 years after initiation of antiarrhythmic therapy. The mean time from ICD implantation to flecainide introduction was 6.1 years.
Of the 11 patients taking flecainide after ICD implantation, 5 (45.5%) had some type of arrhythmic event (symptoms or appropriate ICD shocks) before flecainide introduction and 1 (9%) after its introduction.
DISCUSSIONThe therapeutic arsenal for CPVT currently comprises beta-blockers, ICDs, and flecainide and sympathectomy, 2 recent additions.4 No treatment is completely effective or without risks.
Flecainide is a class IC antiarrhythmic drug with proven efficacy in CPVT.15 The main mechanism of action of flecainide involves selective blockade of the fast inward sodium current in cardiac cells and inhibition of K+ channel opening (particularly that of IKr channels).15 However, because these actions are insufficient to explain its effectiveness in CPVT, additional mechanisms may also be involved, such as open state block of RyR2 channels, which reduces the spontaneous release of calcium from the sarcoplasmic reticulum.16,17
Our study shows that flecainide reduces VAs, symptoms, and ICD shocks in patients with RyR2 genotype-positive CPVT under treatment with beta-blockers. These results are important because 15% of our patients continued to have symptoms despite beta-blocker therapy.13
The main indication for the addition of flecainide to patients’ therapy was persistent complex VAs in follow-up ETs despite beta-blocker therapy (72.2%), a rate that is somewhat similar to the 78% rate reported previously.8
Flecainide was 100% safe in our cohort. No patient abandoned treatment due to adverse effects. This level of safety was reported previously in a series of patients with genotype-negative CPVT, with no patients abandoning treatment due to adverse effects18; however, in the series reported by Van der Werf et al.14, 1 patient (3%) discontinued flecainide therapy due to side effects17, and in the series of Roston et al.8, treatment was discontinued in 5 patients (10%) due to persistent or increasing side effects in a 1.3-year follow-up (vs 2.63 years in our cohort).
Addition of flecainide to beta-blockers was effective from clinical and arrhythmogenic points of view. Previously, up to 53% of patients treated with added flecainide were asymptomatic and 38% continued to have VAs8, higher rates than those obtained in our series.
Although the arrhythmia burden can vary in the same patient with CPVT from one ET to another even without treatment modification14, ET results can be related to future cardiac events19, which is why ETs are usually used to guide treatment in clinical practice. In the patients from family 1, flecainide significantly reduced the quantitative VA score and 66.7% had a greater than 50% reduction in ET-induced VAs. Van der Werf et al.14 reported a 75% reduction in VAs in ETs in 33 patients (similar to our cohort).
As shown in Figure 2, in family 1, flecainide added to beta-blockers significantly decreased the maximum HR without modifying the HR at the onset of the first VA and thereby significantly reduced the arrhythmic window. The HR reduction could inhibit delayed afterdepolarizations, whose amplitude is directly related to HR20, as well as decreasing the HR range during which VAs can occur and, consequently, reducing the risk of more severe VAs.6 However, although this effect was previously seen in a comparison of nadolol with beta1-selective beta-blockers6, the exact causal mechanism is unknown, with other actions of flecainide probably explaining this reduction in exercise-induced arrhythmias.
Leren et al.6 showed that nadolol, compared with beta1-selective beta-blockers, significantly reduced the maximum HR, arrhythmic window, and Van de Werf et al.14 qualitative arrhythmia score (the same qualitative scoring system used in our study). Although the data cannot be directly compared due to methodological differences between the studies, Table 3 shows the values obtained in the series reported by Leren et al.6 and in family 1.
Summary of the Data Obtained in the Exercise Test of the Series Reported by Leren et al.6 and Our Cohort.
| Leren et al.6, beta1 selective* | Leren et al.6, Nadolol* | Family 1, BBs alone | Family 1, BBs + flecainide | |
|---|---|---|---|---|
| Resting HR, bpm | 56 ± 14 | 54 ± 10 | 62 ± 9 | 61 ± 13 |
| Maximum HR, bpm | 139 ± 24 | 122 ± 21 | 144 ± 16 | 128 ± 19 |
| HR PVCs, bpm | 113 ± 19 | 113 ± 21 | 124 ± 26 | 122 ± 18 |
| Arrhythmic window, bpm | 32 ± 26 | 17 ± 10 | 19 ± 21 | 5 ± 11 |
| Qualitative VA score | 2.5 ± 0.8 | 1.6 ± 0.9 | 1.6 ± 1.1 | 1.1 ± 0.5 |
BB, beta-blockers; HR, heart rate; HR PVC, HR at the occurrence of premature ventricular contractions; VAs, ventricular arrhythmias.
Data are presented as mean ± standard deviation.
In the series reported by Leren et al.6, the patients being treated with nadolol had similar qualitative scores and arrhythmic windows to those of family 1 treated with beta-blockers alone. Of the 13 patients in family 1 analyzed, 10 (77%) were treated with propranolol, a nonselective beta-blocker similar to nadolol, which may explain the similar scores. The resting HR was lower in the group reported by Leren et al.6, possibly due to lower beta-blockade, although this is not suggested by the evidence, or because the patients from family 1 with a RyR2 mutation had a higher resting HR than did patients with other mutations in this gene, a possibility that remains to be studied. The patients in our cohort were taking the maximum tolerated dose of beta-blocker and, in fact, all achieved a HR < 85% of the age-predicted maximum heart rate in the ETs—7 of them (54%) ≤ 80%—, indicating good beta-blockade. Our cohort also showed a higher HR at the occurrence of the first premature ventricular contraction than in the cohort reported by Leren et al.6, both in the beta-blocker alone group and in those treated with added flecainide. This finding could also be because the HR at the occurrence of the first premature ventricular contraction was higher in family 1, perhaps due to the type of mutation, although this possibility, as with the resting HR finding discusses above, has not been studied. If nadolol decreases the arrhythmic window vs beta1-selective beta-blockers, it remains to be shown that combination beta-blockers, mainly propranolol and flecainide, can reduce it even further.
Implantable cardioverter defibrillator shocks, both appropriate and inappropriate, should be avoided or reduced in patients with CPVT because they can generate more arrhythmias by increasing sympathetic tone, which can trigger an arrhythmic storm culminating in death.10,12 In our series of patients with an ICD, the use of combined flecainide and beta-blockers reduced the number of shocks and symptoms.
This finding could be important when evaluating patients whose indication for ICD implantation is persistent ET-induced VAs because this approach could reduce ICD indications and the number of shocks and symptoms in patients with an ICD, avoiding undesired effects from both appropriate and inappropriate shocks and decreasing costs and possible complications.
In contrast to most studies, no patients had fatal sudden cardiac death in our cohort. This could indicate the need for a uniform diagnostic protocol for all families that includes genetic screening and universal treatment with beta-blockers, preferably nonselective, titrated with serial ETs.13
The effort expended in trying to reach the maximum beta-blocker dose can mean that patients fail to adhere to the treatment or discontinue it. About 5% of sudden cardiac deaths in patients with CPVT are estimated to be due to treatment nonadherence.17 Combined high-dose flecainide (5mg/kg) and low-dose beta-blockers, applied to minimize adverse effects and improve treatment adherence, has shown clinical efficacy and VA suppression in the literature.17
The optimal dosage of flecainide and beta-blockers remains to be defined but previous studies have reported a dose-response effect for flecainide and that a dosage of 150 to 300mg/d is optimal to reduce VAs, with a dose < 100mg being insufficient.14 In our series, the mean flecainide dose required to reduce the VA quantitative score by more than 50% was 123.8mg and the maximum dose was 200mg. Thus, the doses of both beta-blockers and antiarrhythmic agents should be optimized with serial ETs to limit their dosages as much as possible.
LimitationsThe number of patients who received combined flecainide and beta-blockers or flecainide alone was small. Few studies have examined their combination, and all were retrospective and with few patients14,18, but an ongoing clinical trial is currently testing the efficacy of flecainide in patients with CPVT vs placebo (ClinicalTrials.gov ID: NCT01117454).
To evaluate the VAs, we chose the ET before flecainide initiation and the first after the maximum dose was reached. VAs can differ in the same patient between ETs before flecainide initiation and those performed with maximum flecainide dose, even without a change in beta-blocker dose.
All patients in our cohort had mutations in the RyR2 gene, and analysis of VAs and the arrhythmic window in ETs before and after the addition of flecainide was largely performed in family 1, whose members have a heterozygous missense mutation in Gly357Ser. Accordingly, careful interpretation of the data is required because the findings are not necessarily generalizable to patients with genotype-negative CPVT or with other pathogenic CPVT mutations. Not all RyR2 mutations have the same behavior, and different mutations in RyR2 are believed to be linked to different disease mechanisms and to differently affect flecainide action.21
Finally, families 2 and 7 were studied without a defined follow-up and treatment protocol.
CONCLUSIONSOur study shows that the addition of flecainide to beta-blocker therapy is well-tolerated and effective and reduces ET-induced VAs, the arrhythmic window, and the maximum HR and symptoms and ICD shocks in patients with RyR2-positive CPVT.
FUNDINGImplantable Cardioverter Defibrillator Working Group Grant: “Flecainide therapy in ICD recipients with catecholaminergic polymorphic ventricular tachycardia”.
CONFLICTS OF INTERESTNone declared.
- –
Recent clinical practice guidelines have incorporated the use of flecainide in the treatment of patients with CPVT. Its use is recommended in patients with CPVT who, despite optimal beta-blocker therapy (or without beta-blockers due to intolerance), remain symptomatic, have appropriate or inappropriate ICD shocks, and develop complex VAs in exercise testing (IIA-C indication).
- –
Our study shows that flecainide is safe and effective in patients with genotype-positive CPVT and even safer than in previous studies. This is the first study to analyze the arrhythmic window and quantitative VA burden in ETs in patients under treatment with beta-blockers alone vs beta-blockers and flecainide. Our findings show reductions in VAs, maximum HR, and the arrhythmic window in ETs and in repeated ICD shocks with combined flecainide and beta-blockers.