We explored the differences between atherosclerotic burden with invasive coronary angiography and virtual monochromatic imaging derived from dual-energy computed tomography coronary angiography.
MethodsEighty consecutive patients referred for invasive coronary angiography underwent dual-energy computed tomography coronary angiography and were categorized according to the atherosclerotic burden extent using the modified Duke prognostic coronary artery disease index, coronary artery disease extension score, segment involvement score, and the segment stenosis score.
ResultsThe mean segment involvement score (8.2 ± 3.9 vs 6.0 ± 3.7; P < .0001), modified Duke index (4.33 ± 1.6 vs 4.0 ± 1.7; P = .003), coronary artery disease extension score (4.84 ± 1.8 vs 4.43 ± 2.1; P = .005), and the median segment stenosis score (13.5 [9.0-18.0] vs 9.5 [5.0-15.0]; P < .0001) were significantly higher on dual-energy computed tomography compared with invasive angiography. Dual-energy computed tomography showed a significantly higher number of patients with any left main coronary artery lesion (46 [58%] vs 18 [23%]; P < .0001) and with severe proximal lesions (0.28 ± 0.03 vs 0.26 ± 0.03; P < .0001) than invasive angiography. Levels of coronary artery calcification below and above the median showed a sensitivity, specificity, positive predictive value, and negative predictive value of 100% and 97%; 86% and 50%; 93% and 95%; 100% and 67% for the identification of ≥ 50% stenosis.
ConclusionsDual-energy computed tomography coronary angiography identified a significantly larger atherosclerotic burden compared with invasive coronary angiography, particularly involving the proximal segments.
Keywords
The poor correlation between lesion severity and clinical outcome has been described in a recent study involving a large cohort of patients who underwent invasive coronary angiography (ICA), showing that nonobstructive coronary atherosclerosis is related to a significant increment in the risk of myocardial infarction and all-cause mortality.1 Mounting evidence mostly involving intravascular ultrasound confirmed that the extent and severity of coronary atherosclerosis is usually underestimated by ICA.2,3
Computed tomography coronary angiography (CTCA) has emerged as an accurate method to evaluate coronary atherosclerosis, not only in the lumen but also of the vessel wall. Indeed, CTCA is more closely related to intravascular ultrasound than to ICA and has been shown to provide a significant prognostic value, with an excellent long-term event-free safety window in patients with normal coronary arteries.4,5 Since the emergence of CTCA, coronary calcification has persisted as a dilemma since it often leads to overestimation of stenosis due to several technical issues such as blooming and beam hardening effects. Virtual monochromatic imaging derived from dual-energy (DE) CTCA has emerged as a novel approach that aims to more accurately assess coronary plaques since it attenuates some of these limitations.6 We therefore sought to explore the differences between atherosclerotic burden with ICA and DE-CTCA in symptomatic patients referred for ICA.
METHODSStudy PopulationThe present was a single-center, investigator driven, prospective study that involved patients with suspected coronary artery disease (CAD) referred for ICA. Between May and October 2014, consecutive symptomatic patients referred for ICA who accepted to undergo DE-CTCA within 1 month before the invasive procedure were included in the study. DE-CTCA in patients referred for ICA was encouraged within this period to gain experience in recently acquired DE imaging, although our department has more than 12 years of experience in CTCA. All patients were more than 18 years old, in sinus rhythm, and able to maintain a breath-hold for 15seconds; none had a history of contrast related allergy, renal failure, or hemodynamic instability. Additional exclusion criteria comprised a history of previous myocardial infarction within the previous 30 days, percutaneous coronary intervention with stent implantation, coronary bypass graft surgery, or chronic heart failure.
Image AcquisitionPatients were scanned using a 64-slice high definition scanner (Discovery HD 750, GE Healthcare, Milwaukee, United States), after intravenous administration of iodinated contrast (iobitridol, Xenetix 350, Guerbet, France). A total of 60 to 80mL of iodinated contrast was injected using a 3-phase injection protocol. Image acquisition was performed after sublingual administration of 2.5 to 5mg of isosorbide dinitrate. Patients with a heart rate of more than 65 bpm received 5mg intravenous propranolol, if needed, to achieve a target heart rate of less than 60 bpm.
All studies were acquired using prospective electrocardiogram-gating by applying a 100 msec padding centered at 75% of the cardiac cycle for patients with a heart rate lower than 60 bpm, a 200 msec padding centered at 60% of the cardiac cycle for patients with a heart rate between 60 and 74 bpm, and a 100 msec padding centered at 40% of the cardiac cycle for patients with a heart rate higher than 74 bpm. DE-CTCA was performed by rapid switching (0.3-0.5 msec) between low and high tube potentials (80-140kV) from a single source, thereby allowing the reconstruction of low- and high-energy projections and generation of monochromatic image reconstructions ranging from 40 to 140 keV (kiloelectron volt). Iterative reconstruction was performed in all cases at 40% adaptive statistical iterative reconstruction. Other scanner-related parameters were a collimation width of 0.625mm and a slice interval of 0.625mm.
Image AnalysesAll CTCA image analyses were performed off-line on a dedicated workstation, using a commercially available dedicated software tool (AW 4.6, GE Healthcare) by consensus of 2 experienced level 3–certified coronary CTCA observers (P. Carrascosa and A. Deviggiano), blinded to the clinical data.
Axial planes, curved multiplanar reconstructions, and maximum intensity projections were used at 1-5mm slice thickness, according to the 16-segment modified American Heart Association classification.7,8 We did not use the 18-segment Society of Cardiovascular Computed Tomography classification since we aimed to use the same classification applied in the study of Min et al.9 Whenever plaques were identified, thin multiplanar reconstructions and orthogonal views were obtained at independent monochromatic energy levels ranging from 60 keV to 100 keV to attenuate the beam hardening and blooming artifacts commonly present in calcified plaques, with incremental levels of 10 keV. However, all energy levels were available to the observers. Segments with a reference diameter lower than 1mm were not included in the analysis. Each segment was graded as follows: normal, mild stenosis (< 50%), moderate stenosis (50%-69%), severe stenosis (≥ 70%), or uninterpretable. Uninterpretable segments due to motion artifacts or severe concentric calcification were assumed to be positive for the diagnostic performance analysis and were excluded for the anatomic burden analysis. The coronary artery calcium score (CAC) was calculated by an independent observer (G.A. Rodríguez-Granillo) using the Agatston method with dedicated software (SmartScore; GE Healthcare), which automatically defined the presence of calcified lesions as those with > 130 Hounsfield units (HU).10
The CT effective radiation dose was derived by multiplying the dose-length product with the weighting (k) value of 0.014 mSv/mGy/cm for chest examinations, as suggested by the Society of Cardiovascular Computed Tomography.11
Atherosclerotic Burden ScoresIn keeping with established standard definitions of flow-limiting stenoses and since we used ICA as the criterion standard, nonobstructive CAD was defined as a stenosis ≥ 20% but less than 50% in the left main coronary artery, or a stenosis ≥ 20% but less than 70% in any other epicardial coronary artery. Obstructive CAD was defined as any stenosis ≥ 50% in the left main coronary artery, ≥ 70% in any other coronary artery, or both. Angiograms with absence of stenoses ≥ 20% and/or of mild luminal irregularities were considered normal.12
Subsequently, patients were categorized according to the atherosclerotic burden extent (CAD score). For this purpose, patients were initially classified regarding CAD severity as having single, double, or triple-vessel distribution. Vessel distribution was defined as left anterior descending artery and its tributaries, the left circumflex artery and its tributaries, and the right coronary artery and its tributaries. Patients with isolated 20%-49% left main coronary artery stenosis were recorded as having 1-vessel nonobstructive CAD, whereas those with ≥ 50% left main coronary artery stenosis were recorded as having 3-vessel obstructive CAD patients. For each vascular distribution, we determined the maximal stenosis present and classified the distribution as normal, nonobstructive CAD, or obstructive CAD. As previously established by Maddox et al,1 we created 7 categories of CAD extent: normal; 1-, 2-, and 3-vessel nonobstructive CAD; and 1-, 2-, and 3-vessel obstructive CAD.
Furthermore, atherosclerotic burden was also classified according to the previously reported modified Duke prognostic CAD index as follows: 1, normal; 2, < 50% stenosis; 3, ≥ 2 nonobstructive stenoses (including 1 artery with proximal disease or 1 artery with 50%-69% stenosis); 4, 2 vessels with stenoses 50%-69% or 1 vessel with ≥ 70% stenosis; 5, 3-vessel disease with stenoses 50%-69%, or 2 vessels ≥ 70%, or proximal left anterior descending stenosis ≥ 70%; 6, 3-vessel disease with stenoses ≥ 70% or 2-vessel disease ≥ 70% with proximal left anterior descending; and 7, left main stenosis ≥ 50%.9
Atherosclerotic burden scores were consequently assembled as described by Min et al9: a) segment stenosis score (SSS); b) segment involvement score (SIS), and c) 3-vessel plaque score. Briefly, the SSS, a measure of the overall atherosclerotic burden, where each coronary segment was graded as having no to severe plaque (scores 0 to 3) based on the degree of coronary stenosis as mentioned above. Subsequently, the scores of all segments were summed, leading to a total score ranging from 0 to 48. The SIS reflected the total number of segments involved, irrespective of the degree of stenosis, ranging from 0 to 16. Lastly, a binary score reflecting the absence or presence of 3-vessel plaque was built.9
Invasive Angiography Acquisition and AnalysesAll procedures were performed in accordance with standard techniques. Coronary angiograms were obtained in multiple projections after administration of intracoronary nitrates. Quantitative coronary angiography analysis was performed by an experienced interventional cardiologist blinded to the CTCA data (A. Goldsmit). The catheter tip was cleared of contrast for accurate calibration. Lesion measurements were performed using the “worst” view of an end-diastolic frame.
The institutional review board approved the study protocol, which complied with the Declaration of Helsinki, and written informed consent was obtained from all patients.
Statistical AnalysisDiscrete variables are presented as counts and percentages. Continuous variables are presented as means ± standard deviation and as median [interquartile range], as indicated. Comparisons among groups were performed using paired sample t-tests or nonparametric tests (Wilcoxon signed rank test) for continuous variables with and without normal distribution, respectively. McNemar's test was used for comparisons between paired categorical variables. On the basis of an interim analysis showing that the mean SIS was 8.0 with DE-CTCA and 6.8 with ICA, we calculated a sample size of 60 paired participants to achieve a power of 85% to detect a true difference in population means, considering a type I error of 0.05 (2-sided) and a within-group standard deviation of 3.1. We calculated the sensitivity, specificity, negative predictive value, positive predictive value, and likelihood ratios, accounting for potential nonuniform distribution (95% confidence intervals) of DE-CTCA to identify obstructive CAD. Spearman correlation coefficients were used to explore nonparametric correlations. All statistical analyses were performed using SPSS software, version 22 (Chicago, Illinois, United States). A 2-sided P < .05 indicated statistical significance.
RESULTSEighty patients were prospectively included in the study protocol. The mean age was 62.0 ± 10.9 years, 59 (74%) patients were male, 16 (20%) had diabetes, 57 (71%) had hypertension, and 54 (68%) had hypercholesterolemia. The clinical presentation was typical chest pain in 50 (63%) patients, dyspnea on exertion in 14 (18%) patients, and atypical symptoms with positive stress test in 16 (20%) patients. The mean heart rate was 63.1 ± 8.4 bpm, and the mean effective radiation dose of DE-CTCA alone was 4.4 ± 1.3 mSv. The median Agatston CAC score was 492.0 [interquartile rate, 119.0-1061.5].
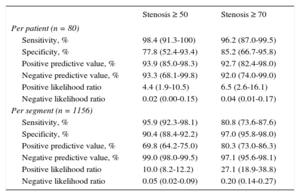
A total of 1156 segments were evaluated with DE-CTCA and ICA. Three segments (0.3%) were deemed nonassessable by DE-CTCA due to motion artifacts or severe concentric calcification. As prespecified on a per protocol basis, these segments were excluded from the atherosclerotic burden analysis but were included and classified as ≥ 50 stenosis for the diagnostic performance analysis. Eleven (14%) patients had normal ICA, with an SIS of zero. Of those, 6 (43%) had evidence of disease on DE-CTCA, although all were mild lesions, with a mean SIS and SSS of 1.5 ± 0.8. On ICA, 62 (78%) patients had at least 1 stenosis larger than 50% compared with 65 (81%) patients on DE-CTCA. Sensitivity, specificity, positive and negative predictive value, and positive and negative likelihood ratio values of DE-CTCA for the detection of obstructive CAD are depicted in Table 1. Patients were further categorized according to CAC median. Patients with CAC below the median showed a sensitivity, specificity, positive predictive value, and negative predictive value of 100% (95%CI, 87%-100%), 85.7% (95%CI, 57%-98%), 92.9% (95%CI, 77%-99%), 100% (95%CI, 74%-100%) for the identification of ≥ 50% stenosis. Patients with extensive calcification (CAC above median) showed a sensitivity, specificity, positive predictive value, and negative predictive value of 97.2% (95%CI, 85%-100%), 50.0% (95%CI, 7%-93%), 90.0% (95%CI, 76%-97%), 66.7% (95%CI, 9%-99%) for the identification of ≥ 50% stenosis.
Diagnostic Performance of Dual-energy Computed Tomography Coronary Angiography on a Per Patient and Per segment Level
| Stenosis ≥ 50 | Stenosis ≥ 70 | |
|---|---|---|
| Per patient (n = 80) | ||
| Sensitivity, % | 98.4 (91.3-100) | 96.2 (87.0-99.5) |
| Specificity, % | 77.8 (52.4-93.4) | 85.2 (66.7-95.8) |
| Positive predictive value, % | 93.9 (85.0-98.3) | 92.7 (82.4-98.0) |
| Negative predictive value, % | 93.3 (68.1-99.8) | 92.0 (74.0-99.0) |
| Positive likelihood ratio | 4.4 (1.9-10.5) | 6.5 (2.6-16.1) |
| Negative likelihood ratio | 0.02 (0.00-0.15) | 0.04 (0.01-0.17) |
| Per segment (n = 1156) | ||
| Sensitivity, % | 95.9 (92.3-98.1) | 80.8 (73.6-87.6) |
| Specificity, % | 90.4 (88.4-92.2) | 97.0 (95.8-98.0) |
| Positive predictive value, % | 69.8 (64.2-75.0) | 80.3 (73.0-86.3) |
| Negative predictive value, % | 99.0 (98.0-99.5) | 97.1 (95.6-98.1) |
| Positive likelihood ratio | 10.0 (8.2-12.2) | 27.1 (18.9-38.8) |
| Negative likelihood ratio | 0.05 (0.02-0.09) | 0.20 (0.14-0.27) |
95%CI, 95% confidence interval.
Dual-energy CTCA identified a significantly larger atherosclerotic burden compared with ICA as reflected by all explored indexes (Figure 1 and Figure 2). Both the mean SIS (8.2 ± 3.9 vs 6.0 ± 3.7; P < .0001) and the median SSS (13.5 [9.0-18.0] vs 9.5 [5.0-15.0]; P < .0001) were significantly higher on DE-CTCA compared with ICA. Sixty-six (83%) patients with DE-CTCA had evidence of 3-vessel plaque (any degree) compared with 52 (65%) patients with ICA (P < .0001). Furthermore, DE-CTCA showed a significantly higher number of patients with any left main coronary artery lesion (46 [58%] vs 18 [23%]; P < .0001) and with severe proximal lesions (0.28 ± 0.03 vs 0.26 ± 0.03; P < .0001) than ICA (Table 2).
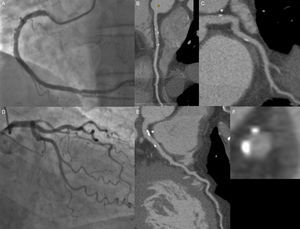
A 59-year-old man with hypertension as a coronary risk factor and typical chest pain. On invasive coronary angiography (panels A and D), mild lesions are observed in the mid and distal right coronary artery; in contrast, on computed tomography coronary angiography, mild predominantly calcified lesions are identified in the proximal, mid, and distal right coronary artery (panel B), left main coronary artery (asterisk, panels C, E, and F), proximal left circumflex (panel C), and proximal left anterior descending (panel E).
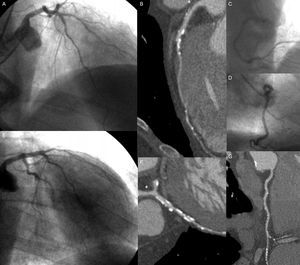
An 82-year-old man with diabetes and hypertension as coronary risk factors and indication of invasive coronary angiography. On invasive coronary angiography, mild lesions are seen in the mid left anterior descending coronary artery (panels A and E), in the mid left circumflex (panel E), and in the distal right coronary artery (panel C). Computed tomography coronary angiography revealed a moderate calcified lesion in the mid left anterior descending artery, with mild proximal and distal lesions (panel B), a severe lesion in the obtuse marginal branch (panel F), and mild calcified lesions among all segments of the right coronary artery (panel G). Furthermore, a mild noncalcified plaque is observed in the left main coronary artery (asterisk).
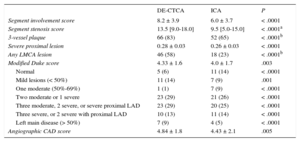
Anatomic Plaque Burden Scores Assessed by DE-CTCA and ICA
| DE-CTCA | ICA | P | |
|---|---|---|---|
| Segment involvement score | 8.2 ± 3.9 | 6.0 ± 3.7 | < .0001 |
| Segment stenosis score | 13.5 [9.0-18.0] | 9.5 [5.0-15.0] | < .0001a |
| 3-vessel plaque | 66 (83) | 52 (65) | < .0001b |
| Severe proximal lesion | 0.28 ± 0.03 | 0.26 ± 0.03 | < .0001 |
| Any LMCA lesion | 46 (58) | 18 (23) | < .0001b |
| Modified Duke score | 4.33 ± 1.6 | 4.0 ± 1.7 | .003 |
| Normal | 5 (6) | 11 (14) | < .0001 |
| Mild lesions (< 50%) | 11 (14) | 7 (9) | .001 |
| One moderate (50%-69%) | 1 (1) | 7 (9) | < .0001 |
| Two moderate or 1 severe | 23 (29) | 21 (26) | < .0001 |
| Three moderate, 2 severe, or severe proximal LAD | 23 (29) | 20 (25) | < .0001 |
| Three severe, or 2 severe with proximal LAD | 10 (13) | 11 (14) | < .0001 |
| Left main disease (> 50%) | 7 (9) | 4 (5) | < .0001 |
| Angiographic CAD score | 4.84 ± 1.8 | 4.43 ± 2.1 | .005 |
CAD, coronary artery disease; DE-CTCA, dual-energy computed tomography coronary angiography; ICA, invasive coronary angiography; LAD, left anterior descending; LMCA, left main coronary artery.
The modified Duke prognostic CAD index (4.33 ± 1.6 vs 4.0 ± 1.7; P = .003) as well as the recently reported CAD extension score (4.84 ± 1.8 vs 4.43 ± 2.1; P = .005) were significantly higher on DE-CTCA compared with ICA.
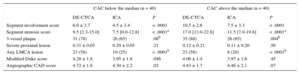
Finally, patients were categorized according to the median CAC score and the differences in atherosclerotic burden scores between DE-CTCA and ICA were evaluated (Table 3), showing a larger extent of atherosclerotic burden irrespective of the degree of calcification. In particular, large differences were observed regarding the presence of any plaque in the left main coronary artery. Nonetheless, the relationships between CAC and both the SIS (CAC below median r = 0.64, P < .0001; CAC above median r = 0.21; P = .20) and the SSS (CAC below median r = 0.62; P < .0001; CAC above median r = 0.30; P = .06) were not significant in patients with severe calcification.
Anatomic Plaque Burden Scores Assessed by DE-CTCA and ICA According to the Extension of CAC
| CAC below the median (n = 40) | CAC above the median (n = 40) | |||||
|---|---|---|---|---|---|---|
| DE-CTCA | ICA | P | DE-CTCA | ICA | P | |
| Segment involvement score | 6.0 ± 3.7 | 4.5 ± 3.4 | < .0001 | 10.5 ± 2.6 | 7.5 ± 3.3 | < .0001 |
| Segment stenosis score | 9.5 [2.3-15.0] | 7.5 [0.0-12.8] | < .0001a | 17.0 [13.0-22.8] | 11.5 [7.0-19.8] | < .0001a |
| 3-vessel plaque | 31 (78) | 26 (65) | .06b | 35 (88) | 26 (65) | .004b |
| Severe proximal lesion | 0.31 ± 0.05 | 0.29 ± 0.05 | .21 | 0.12 ± 0.21 | 0.11 ± 0.20 | .56 |
| Any LMCA lesion | 23 (58) | 10 (25) | < .0001b | 23 (58) | 8 (20) | < .0001b |
| Modified Duke score | 4.28 ± 1.8 | 3.95 ± 1.8 | .046 | 4.06 ± 1.4 | 3.97 ± 1.6 | .45 |
| Angiographic CAD score | 4.72 ± 1.8 | 4.30 ± 2.2 | .02 | 4.83 ± 1.7 | 4.46 ± 2.1 | .07 |
CAC, coronary artery calcium score; CAD, coronary artery disease; DE-CTCA, dual-energy computed tomography coronary angiography; ICA, invasive coronary angiography; LMCA, left main coronary artery.
Overall, DE-CTCA assessment identified a larger extent of disease in the proximal and midcoronary segments compared with ICA (Figures 1 and 2 of the supplementary material). In particular, CAD extent was significantly higher in the left main coronary artery (DE-CTCA 0.69 ± 0.7 vs ICA 0.29 ± 0.6; P < .0001) and in the proximal left anterior descending artery (DE-CTCA 1.34 ± 1.0 vs 1.05 ± 1.0; P < .0001), whereas no significant differences were observed in the distal left anterior descending artery (DE-CTCA 0.52 ± 0.8 vs ICA 0.44 ± 0.8; P = .16).
DISCUSSIONOur findings can be summarized as follows: a) DE-CTCA identified a significantly larger atherosclerotic burden than did ICA; b) DE-CTCA had a high sensitivity and specificity in ruling out obstructive CAD in symptomatic patients at intermediate to high probability of CAD; c) DE-CTCA identified a larger extent of disease in proximal and midcoronary segments than did ICA, particularly in the left main coronary artery and the proximal left anterior descending artery.
Several studies including both ICA and CTCA have reported significant discrepancies in the physiological impact of epicardial stenosis severity on downstream myocardial ischemia.13,14 This inconsistency might be partially attributed to the fact that coronary arteries with mild disease usually have abnormal coronary endothelial-dependent vasoreactivity. In fact, Naya et al14 reported that the extent of atherosclerosis, as assessed by the modified Duke CAD index, was associated with decreased myocardial flow reserve.
In our study, we demonstrated a significantly larger atherosclerotic burden by using DE-CTCA than by using ICA. Notably, the advent of spectral imaging enabling synthesized monochromatic image reconstructions (DE-CTCA) has been shown to attenuate a number of artifacts related to coronary calcifications, such as beam hardening and blooming effects.6 Phantom model studies and some clinical data suggest that DE-CTCA, by means of the aforementioned ability to reduce artifacts, might lead to an improvement in the assessment of the degree of coronary stenosis and even to a better discrimination of atherosclerotic plaque characterization.15–19
Diagnostic PerformancePatients with an intermediate to high likelihood of CAD such as our population are commonly excluded from most clinical CTCA studies. Some reports are worth mentioning to put our diagnostic performance results in perspective, despite obvious differences in acquisition protocols, technologies, and populations. In one of the first studies, Meijboom et al20 reported a sensitivity of 90%, specificity 90%, positive predictive value 56%, and negative predictive value of 98% for conventional CTCA in patients at high likelihood of CAD. More recently, in a relatively similar population, Schaap et al21 reported a sensitivity of 98%, specificity 62%, positive predictive value of 77%, and negative predictive value of 96%.
To the best of our knowledge, Carrascosa et al22 performed the only direct comparison between the 2 techniques, although it should be stressed that in that study DE-CTCA was performed with a 50% iodine load whereas conventional CTCA was performed with a full iodine load. In this study, no significant differences were observed either in mean effective radiation dose (3.5 ± 1.9 mSv vs 3.8 ± 0.9 mSv; P = .48), or in sensitivity or specificity (DE-CTCA 84%, 87.1%; conventional CTCA 84.4%, 87.1%). In the present study, although DE-CTCA had a high overall sensitivity and specificity in ruling out significant CAD, the diagnostic performance was lower among patients with extensive calcification, although it is worth mentioning that those patients had a very high prevalence of obstructive CAD.
Atherosclerotic BurdenAll explored indexes included in the present investigation showed significant differences between the 2 methods; namely the modified Duke CAD index, the CAD extension score, and the SSS—which reflect the extension and severity of atherosclerotic burden—, as well as the SIS—which only reflects the extent of the atherosclerotic burden. Furthermore, DE-CTCA identified a significantly higher number of patients with 3-vessel plaque, with severe proximal lesions, and with significant left main coronary artery disease. It is remarkable that of the 11 patients with normal ICA, 43% had evidence of disease on DE-CTCA, although all lesions were mild. In contrast, on ICA, 78% patients had at least one stenosis larger than 50% compared with 81% patients on DE-CTCA, underscoring that the underestimation of atherosclerotic burden by ICA is found mostly in segments with minimal disease.
In a recent subanalysis of the PROSPECT study, residual angiographically mild lesions were a common finding after percutaneous coronary intervention for acute coronary syndromes, and conferred a higher rate of recurrent ischemic events within 3 years.23 Interestingly, in a recent large retrospective ICA cohort published by Maddox et al,1 the authors reported that the risk of myocardial infarction progressively increased according to the CAD extent rather than abruptly increasing between nonobstructive and obstructive CAD. Indeed, patients with nonobstructive CAD had significantly higher rates of myocardial infarction and death than those with normal ICA.1 Furthermore, in a large cohort of patients with very long follow-up, Ostrom et al24 demonstrated that the burden of CAD detected by electron-beam CTCA was significantly related to the incidence of all-cause mortality among symptomatic patients with suspected CAD and progressively increased in keeping with increments in the CAD extent.
Finally, in our study, we identified a larger extent of disease in proximal and midcoronary segments compared with ICA. This finding deserves consideration for further investigations since lesions in these locations usually portend a worse lesion phenotype.25,26 In contrast, the lack of difference in atherosclerotic burden among the distal segments might potentially be adjudicated to the combination of a much lesser extent of disease in those segments among vessels with significantly reduced size, hence with a lower sensitivity to detect plaques by CTCA.
This is the first prospective study to compare different atherosclerotic burden indexes using ICA and DE-CTCA, which is a technology that shows promise in attenuating a number of artifacts related to coronary calcifications that are relevant for the purpose of this investigation.
Indeed, the significant differences observed in atherosclerotic burden scores between DE-CTCA and ICA persisted in patients with severe calcification (CAC score above the median). Nonetheless, the significant relationship between these scores and the extent of calcification was lost in patients with severe calcification. These findings, in line with the lower diagnostic performance among patients with extensive calcification, suggest that even with the development of novel technologies such as DE, diffuse calcification remains a limitation of the technique.
Overall, our findings provide further insights into the limitations of ICA and the concept of normal or near normal coronary arteries. Although the clinical implications of our findings remain to be established in large prospective outcome investigations, all these variables might potentially bear relevant clinical implications in light of mounting evidence linking mild coronary atherosclerosis with adverse clinical events.
A number of limitations should be acknowledged. The relatively small sample size might lead to selection bias. Furthermore, ICA was not performed using 3-dimensional coronary angiography, which might have offered a better evaluation of the coronary tree, although mostly in terms of vessel foreshortening, hence affecting lesion length rather than the degree of obstruction. We did not perform conventional (single-energy) CTCA in these patients for obvious ethical reasons, considering that the patient population was clinically referred for ICA. Accordingly, although previous studies have shown improved image quality and reduced artifacts with this technique both in coronary imaging and myocardial perfusion analyses, it could not be inferred from our study whether DE-CTCA offers superior estimation of atherosclerotic burden than conventional CTCA.6 We did not attempt to perform any regression analyses involving clinical outcome data or longitudinal analyses. Since we simply sought to explore the differences between atherosclerotic burden with ICA and DE-CTCA, clustering of data does not affect the validity of our findings.
CONCLUSIONSIn this study, CTCA using DE imaging identified a significantly larger atherosclerotic burden than did ICA, particularly involving the proximal segments of the coronary tree. Our findings provide further insights into the limitations of ICA and the concept of normal or near normal coronary arteries, although the potential clinical implications should be explored in further prospective natural history studies.
- -
There is a poor correlation between lesion severity assessed by ICA and clinical outcome. It is increasingly important to assess the extent of nonobstructive coronary atherosclerosis, since it is related to abnormal coronary endothelial-dependent vasoreactivity and to an incremental risk of myocardial infarction and all-cause mortality. Since the emergence of CTCA, coronary calcification has persisted as a dilemma, because it often leads to overestimation of stenosis due to several technical issues. Virtual monochromatic imaging derived from DE-CTCA has emerged as a novel approach that aims to convey a more accurate assessment of coronary plaques.
- -
This is the first prospective study to compare different atherosclerotic burden indexes using ICA and DE-CTCA. Using DE-CTCA, we demonstrated a significantly larger atherosclerotic burden in the proximal segments of the coronary tree compared with ICA. DE-CTCA showed a high sensitivity and specificity in ruling rule out obstructive CAD in symptomatic patients at intermediate to high probability of CAD, a population commonly excluded from most clinical CTCA studies.
P. Carrascosa is a consultant for GE Healthcare.