Coronary computed tomography angiography (CCTA) is gaining widespread acceptance for the noninvasive evaluation of coronary arteries.1,2 Although the overall diagnostic performance of coronary evaluation has been demonstrated to be satisfactory, beam-hardening (BH) artifacts resulting from heavily calcified plaques and motion artifacts due to high heart rate may hinder image quality and significantly reduce coronary evaluability and the diagnostic accuracy of CCTA. Recently, dual-energy computed tomography (DECT) has been proposed to improve the diagnostic performance of CCTA, particularly for the reduction of BH artifacts in patients with severe coronary calcification.3 Briefly, whereas conventional computed tomography (CT) is performed at a fixed tube potential with polychromatic energy levels of photons set to 120 or 140 kVp, DECT allows the acquisition of 2 image datasets of the same anatomical region of interest. This provides information regarding changes in energy-dependent attenuation of tissue when it is exposed to 2 different photon-energy levels, thus improving tissue characterization. Although the concept is similar, there are different manufacturer-specific approaches by which DECT images can be obtained. The exploitation of 2 polychromatic energy spectra by DECT can be achieved by at least 3 different methods: a) 2 x-ray source and detector pairs with each source operating at a different tube voltage; b) a single source-detector pair with an x-ray tube capable of rapidly switching between low and high tube potential or by switching tube potential between gantry positions; and c) an x-ray source operating at constant tube voltage with a double-layer detector capable of differentiating between low- and high-energy photons.3 Advantages and disadvantages have been reported for each of the 3 types of DECT scanners. They include good spectral separation between high- and low-energy scans but limited temporal and spatial registration for dual-source CT, good temporal and spatial registration but limited energy separation with spectral overlap for single-source with dual detector layers, and good temporal registration between high- and low-energy datasets but a higher noise level on images obtained at a lower peak voltage for single-source with fast Kv switching.4 The potential usefulness of DECT for ischemic heart disease was documented in different clinical scenarios, including myocardial BH reduction and image quality improvement in myocardial perfusion imaging,5,6 reduction of coronary artery BH due to calcified plaques,7,8 improvement of myocardial infarction detection with late-phase DECT9 and a>50% reduction of contrast agent administration in CCTA.10 Regarding coronary evaluation, DECT performed with a single-source rapid kilovolt peak-switching scanner allows synthesizing of monochromatic images that can be reconstructed from 40 to 140 keV, mimicking images as if monochromatic x-ray sources were used. With this technique, significant BH reduction and improvements in the contrast-to noise-ratio and signal-to-noise ratio in coronary arteries vs conventional single-energy evaluation was demonstrated,8 particularly when the use of monochromatic images was associated with an iterative reconstruction algorithm instead of traditional filtered back projection.9 Moreover, the use of monochromatic reconstructions appeared to improve quantification of coronary stenosis using quantitative coronary angiography as the criterion standard.11
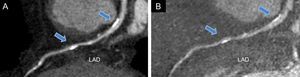
Recently, a single-center study demonstrated a significant improvement of diagnostic accuracy, particularly of the specificity and positive predictive values of DECT performed with monochromatic images and calcium removal by material decomposition imaging in patients with a high (> 400) coronary calcium score.12 The Figure shows an example of calcium removal by material decomposition imaging with DECT. In the article published in Revista Española de Cardiología, Rodríguez-Granillo et al.13 explored the possibility of evaluating, in 80 consecutive patients referred for invasive coronary angiography (ICA) for suspected coronary artery disease (CAD), the extension and spatial distribution of coronary atherosclerotic burden obtained with virtual monochromatic imaging derived from DECT and compared with ICA findings. The main result of the study was that DECT was able to identify a significantly larger atherosclerotic burden than ICA, as reflected by all explored indexes of coronary plaque burden. Indeed, the segment involvement score, which reflects the coronary atherosclerotic burden, counting the number of coronary segments with any grade of plaque, as well as the modified Duke CAD index, the CAD extension score, and the segment stenosis score, which reflect the extension and severity of the atherosclerotic burden, were significantly higher with DECT compared with ICA. These findings, which are in agreement with several previous studies that compared plaque burden evaluation by ICA and intravascular ultrasound and showed a systematic underestimation of the extent and severity of coronary atherosclerosis by ICA,14 appear clinically relevant, particularly concerning their prognostic implications. Indeed, mounting evidence has shown that coronary atherosclerosis extent and the presence of nonobstructive lesions have robust prognostic implications for the prediction of hard cardiac events in both settings: patients with unknown CAD undergoing CCTA and patients undergoing ICA for acute myocardial infarction.15,16 Moreover, the study by Rodríguez-Granillo et al. shows that 43% out of the 11 patients with normal ICA had evidence of disease on DECT, although all lesions were categorized as mild. Therefore, this finding further highlights the limitations of ICA and the weakness of a “normal” coronary angiography.
Left anterior descending coronary artery dual-energy coronary computed tomography angiography imaging performed with standard reconstruction (A) and after calcium removal by material decomposition imaging (Iodine minus hydroxyapatite) (B). The arrows indicate 2 calcified plaques in the proximal and mid portion of the vessel causing beam-hardening artifacts at standard evaluation (A) that were significantly reduced by calcium subtraction (B). LAD, left anterior descending.
Another interesting finding of the study was that DECT showed a significantly higher number of patients with left main coronary artery lesions (58% vs 23%) and a higher number of severe proximal lesions than ICA. This finding deserves consideration and warrants further investigations since lesions at these locations usually portend a worse prognosis. However, the finding should be interpreted with caution because the tendency of the overestimation of severe stenosis by CCTA is a well-known limitation of the method, particularly in patients with a high pretest probability of CAD.17 In addition, although DECT demonstrated an overall good sensitivity and specificity in ruling out significant CAD despite an intermediate-to-high pretest probability of CAD, its diagnostic performance was lower among patients with extensive calcification and a high prevalence of obstructive CAD. This suggests that even with novel technologies such as DECT, diffuse calcifications remain a limitation of CCTA. To overcome these limitations, several studies have documented the incremental diagnostic value of combining anatomical evaluation of coronaries by CCTA with stress-CT myocardial perfusion imaging, when compared with ICA plus single photon emission CT. Of note, the use of DECT instead of single-energy seemed particularly helpful, due to improvement in the image quality of the myocardial perfusion data set related to a significant reduction of myocardial BH.6,7
Finally, since patients only underwent DECT, the study by Rodríguez-Granillo et al. does not identify whether DECT offers superior estimates of atherosclerotic burden and better plaque characterization than conventional CCTA. Nevertheless, another DECT might still provide improved evaluation of plaque characteristics. In this regard, extensive efforts have been made to identify rupture-prone “vulnerable plaques”. From prior invasive and pathologic evaluations, several anatomical characteristics have been implicated as crucial to the pathogenesis of acute coronary syndromes, such as thin cap fibroatheroma, small spotty calcifications and necrotic lipid-rich core.18 Given their importance, these plaque features have been extensively investigated by single-energy CT, due to the relative ease with which single-energy CT separates calcified from noncalcified plaques. However, single-energy CT faces a significant challenge in differentiating the various anatomical components of noncalcified plaques (eg, lipid-rich vs fibrous). Identification of different atherosclerotic plaque components is rather challenging and several studies have shown considerable overlap in Hounsfield units between lipid-rich and fibrous-rich noncalcified plaques attributable to the spatial resolution of CT and a variable interaction between this type of plaque and iodine contrast agents.19 Due to its capability for material decomposition, DECT may overcome these limitations, thus improving plaque differentiation.20 Better plaque characterization by CCTA will be fundamental for patient prognosis stratification, because identification of some plaque features, which have been associated with a high-risk of events, such as vessel-positive remodeling and low-attenuation plaques, have been demonstrated to have an even more powerful value than the degree of stenosis alone for cardiac event prediction.21 In conclusion, the study by Rodríguez-Granillo et al. provides further support for the concept that CCTA, and particularly DECT, are useful diagnostic tools for the noninvasive identification and characterization of atherosclerotic burden in patients with suspected CAD.
CONFLICTS OF INTERESTNone declared.