To evaluate the capability of multidetector computed tomography to diagnose the coronary etiology of left ventricular dysfunction compared with using invasive coronary angiography and magnetic resonance.
MethodsForty consecutive patients with left ventricular dysfunction of uncertain etiology underwent invasive coronary angiography and contrast magnetic resonance. All patients were evaluated with multidetector computed tomography including coronary calcium presence and score, noninvasive coronary angiography, and myocardial tissue assessment.
ResultsThe sensitivity and specificity of the presence of coronary calcium to identify left ventricular dysfunction was 100% and 31%, respectively. If an Agatston calcium score of >100 is taken, specificity increases to 58% with sensitivity still 100%. Sensitivity and specificity for coronary angiography by multidetector computed tomography was 100% and 96%, respectively; for identifying necrosis in contrast acquisition it was 57% and 100%, respectively; and in late acquisition, 84% and 96%, respectively. To identify coronary ventricular dysfunction with necrosis, the sensitivity and specificity was 92% and 100%, respectively.
ConclusionsOf all the diagnostic tools available in multidetector computed tomography, coronary angiography is the most accurate in determining the coronary origin of left ventricular dysfunction. A combination of coronary angiography and myocardial tissue study after contrast allows a single test to obtain similar information compared with the combination of invasive coronary angiography and contrast magnetic resonance.
Keywords
.
INTRODUCTIONGiven the high prevalence of coronary heart disease (CHD) among patients with heart failure (HF) and left ventricular systolic dysfunction (LVSD), the etiological study should exclude underlying CHD.1 HF associated with dilated cardiomyopathy or ischemic cardiomyopathy (IC) may be clinically indistinguishable, especially in patients without symptoms suggestive of angina, necrotic Q waves in the electrocardiogram (ECG), or complete left bundle branch block in the ECG, which impedes identification.2 The definitive diagnosis of IC is based on showing obstructive CHD via coronary angiography. It has a low risk of complications, but these can be serious3; therefore, a noninvasive approach to diagnosis of CHD may be preferable, especially in patients without ischemic symptoms and no history of myocardial infarction.
In these patients, most noninvasive approaches in the etiological diagnosis of the underlying cardiomyopathy in patients with HF and LVSD, such as echocardiography or nuclear medicine studies, require coronary angiography. Thus, although nuclear medicine studies have acceptable sensitivity, especially if combined with a perfusion study and contractility during stress testing, the specificity is very low.4 Furthermore, Doppler echocardiography studies to detect segmental wall motion abnormalities show a similar sensitivity to nuclear medicine studies but with better specificity, especially if dobutamine is used.5, 6 However, the quite frequent presence of left bundle branch block, associated with abnormal septal motion, limits its routine use.
Cardiac magnetic resonance (CMR) imaging combines the study of overall and segmental contractile function (as in Doppler echocardiography and nuclear medicine) and myocardial perfusion (such as nuclear medicine). In addition, late acquisitions after contrast administration allow myocardial segments with areas of necrosis to be seen. Their detection by late gadolinium enhancement (LGE) has demonstrated excellent diagnostic accuracy for identifying patients with HF and underlying CHD, even in patients with a low initial probability of ischemic heart disease.7, 8
There are few studies that assess the diagnostic accuracy of computed tomography in LVSD patients, whether through the identification of coronary calcifications,9, 10 coronary lesions,11, 12, 13, 14 or areas of necrosis in a manner similar to CMR but using iodine compounds as contrast agents.14 However, no study so far has evaluated the diagnostic usefulness of these tools in the same group of patients.
The objectives of this study were to determine the diagnostic value of each available tool in multidetector computed tomography (MDCT) to identify LVSD of coronary origin (detection of coronary calcium, identification of significant coronary lesions, detection of areas of necrosis) and to assess the diagnostic accuracy of combined coronary angiography and the detection of necrosis in the same test using MDCT to identify patients with LVSD of coronary origin, based on invasive angiography data and the detection of necrosis by CMR.
METHODSThis prospective study included 40 patients (24 men and 16 women) with a mean age of 61 years (range 39-85), newly diagnosed with HF of unknown origin, and echocardiographic confirmation of LVSD (ejection fraction<40%) and dilated left ventricle (end-diastolic diameter >95 percentile, depending on the body surface area). Only those patients without known or clinical suspecion of CHD, necrotic Q waves on the ECG, or laboratory data showing significant elevation of myocardial necrosis biomarkers were included. Patients were excluded with other causes of cardiomyopathy: infiltrative cardiomyopathy, hypertrophic cardiomyopathy, or myocarditis, as well as those with significant valve disease. All patients were in sinus rhythm and hemodynamically stable.
Patients with a contraindication for iodine contrast administration, either a history of allergy to iodine contrast or chronic renal failure (serum creatinine >1.5mg/dl), were also excluded. Those unable to undertake magnetic resonance imaging due to having severe claustrophobia, cerebral clips, a pacemaker or defibrillator were also excluded.
The study was approved by the ethics committee of our hospital and all patients gave written informed consent.
Cardiac Magnetic ResonanceA 1.5 Tesla magnet was used (Magnetom Sonata®, Siemens, Erlangen, Germany). The film sequences were obtained with cardiac synchronism and in apnea using steady-state-free precession (SSFP) sequences in long-axis (2, 3, and 4 cameras) and short-axis (slice thickness of 8mm and 2mm of separation between slices) cuts covering the mitral ring to the apex. Gadobenate dimeglumine 0.5M (0.15 mmol/kg) was used as a contrast agent administered in a peripheral vein.
For the perfusion study, a SSFP sequence with cardiac synchronism of at least 4 short-axis and 1 long-axis cuts was used. The images for the study of LGE were obtained using 2D and 3D gradient echo sequences (turbo-FLASH: fast low angle shot), with inversion-recovery pulse at 8 to 10min after administration of the contrast, obtaining short- and long-axis cuts as in the functional study. Inversion time to nullify the myocardial signal and detect areas of LGE was adjusted according to the time elapsed. The SSFP sequences were optionally used as well.
To analyze the LGE, the standard 17-segment myocardial model was used.15
The LGE patterns examined were: a) lack of LGE; b) linear or focal intramyocardial LGE (fibrosis), and c) subendocardial or transmural LGE (necrosis).
Multidetector Computed TomographyA 64-detector scanner with a complete rotation time of 330ms (LightSpeed VCT®, GE. Milwaukee, Wisconsin, United States) was used to obtain 64 slices with a thickness of 0.625mm and 40mm coverage per rotation, which allowed the acquisition of a volume that included the heart at an average apnea time of 6s.
The coronary calcification study was performed (without contrast) with cardiac synchronism: slice thickness of 2.5mm, 120kV and 430mA.
Nonionic iodine contrast (Iomeron® 400, Bracco, Italy) was used for the coronary angiography, with the volume adjusted to the patient's weight, injected through the antecubital vein with an infusion rate of 5ml/s and starting acquisition when the contrast reached the ascending aorta. In general, 70ml was used for patients weighing <70kg and up to 120ml for those weighing >70kg.
The acquisition was synchronized with the ECG using a voltage of between 100kV and 120kV and a 750mA effective current, depending on the body surface. Modulation of the radiation dose was also used (maximum radiation dose in diastolic phases and a reduction for the systole).
Although most patients were on chronic treatment with beta blockers, an additional dose of 1-2.5mg intravenous atenolol was administered in 12 patients due to heart rates above 65 beats/min before the start of the test.
Following acquisition, the cardiac cycle phases were reconstructed retrospectively (0% to 90% with successive increments of 10%) using a segmented algorithm (temporal resolution of 165ms), to obtain reconstructions in the phase with the smaller cardiac motion artifact. Image analysis was performed on an Advantage Work Station 4.3® (General Electric Medical System, Milwaukee, Wisconsin, United States) with specific software for the heart study. For the coronary study, curved MPR (multiplane reformat), MIP (Maximum intensity projection), and 3D-VR (volume rendering) reconstructions were used. The 16-segment model was used.16
Acquisition with contrast was used to reconstruct the coronary tree images, from which long-axis (2, 3, and 4 cameras) and short-axis (base to apex) images were reconstructed in systolic and diastolic phases to identify myocardial areas of signal hypoattenuation (areas of necrosis), using the same model of myocardial segmentation as in CMR.15
Between 8 and 10min after the administration of contrast, the acquisition was repeated (with the same volume) to assess myocardial areas showing late iodine enhancement (LIE). A lower voltage (80kV) was used for this acquisition, with cardiac synchronism and reducing the effective current to a shorter diastole to get a better signal/noise ratio and a decrease in the effective radiation dose. Long-axis (2, 3, and 4 cameras) and short-axis (base to apex) images were reconstructed with a slice thickness of at least 8mm, using a soft tissue window and the same myocardial segmentation described.15
An experienced assessor analyzed all the studies. The estimated average radiation dose for the entire protocol was 1462±274mGy/cm (25±5mSv) using the conversion factor for the chest (0.017 times).
Invasive Coronary AngiographyThe study was subsequently performed with MDCT and CMR (mean interval of 22±10 days) with Coroskop Plus/TOP® (Siemens, Munich, Germany) equipment through a femoral artery puncture and selective coronary catheterization with the usual angiographic images being taken. The image analysis was performed by the hemodynamics lab specialist responsible for the procedure, with the presence of lesions in the vessels being determined visually, in the same way as with MDCT and using the same coronary segment model.16
Definition of Ischemic CardiomyopathyFor the diagnosis of ischemic LVSD (of coronary origin), the coronary angiography results were considered as reference when meeting the criteria established by Felker et al.17: >75% damage to the common trunk of the left coronary artery or the proximal segment of the anterior descending artery, or damage >75% in 2 or 3 vessels.
Diagnosis of Left Ventricular Systolic Dysfunction of Coronary Origin by Multidetector Computer TomographyThe following possibilities for diagnosis of CHD by MDCT were assessed by comparing with the diagnosis of coronary LVSD based on coronary angiography results: the presence of coronary calcification (as an indirect marker of significant coronary lesion), the identification of significant coronary lesions meeting the Felker et al.17 criteria (with contrast), the identification of areas of necrosis as areas of myocardial signal hypoattenuation in the same acquisition as the coronaries, and the use of LIE to identify areas of necrosis in the delayed acquisition by the presence of iodine in the myocardium.
Based on the combined results of coronary angiography-MDCT and LIE, 4 patient groups were established:
• Group 1. Those complying with coronary angiography criteria for ischemic systolic dysfunction and having subendocardial or transmural LIE (necrosis).
• Group 2. Those not meeting LVSD coronary angiography criteria and without subendocardial or transmural LIE, although they may have linear or focal intramyocardial LIE (fibrosis).
• Group 3. Those not meeting LVSD coronary angiography criteria and who have subendocardial or transmural LIE (necrosis).
• Group 4. Those meeting LVSD coronary angiography criteria and who do not have subendocardial or transmural LIE (necrosis).
These 4 groups were compared with the same 4 groups established on the basis of information obtained from invasive coronary angiography and LGE-CMR.
Statistical AnalysisStatistical analysis was performed using the statistical software package for social sciences (SPSS v. 17.0 for Windows, SPSS Inc, Chicago, Illinois, United States).
Normal distribution was checked for the quantitative variables using the Kolmogorov-Smirnov test. All continuous and normal variables were expressed as mean±standard deviation, and the others as percentages.
The accuracy of MDCT in the diagnosis of LVSD was determined by a 2×2 contingency table using conventional invasive coronary angiography as standard. One-off estimates were obtained for sensitivity and specificity, and diagnostic accuracy of the test using the standard inference methods for proportions.18
Similarly, the 4 groups established on the basis of coronary angiography and LGE-RMI results were compared with the 4 groups established by the coronary angiography-MDCT and LIE-MDCT results.
Finally, the correlation between the 2 experienced observers was analyzed using Cohen's Kappa index for the classification of groups.
RESULTSTable 1 shows the characteristics of the population studied.
Table 1. Sample Characteristics (n=40).
| Age, years | 61; 65.7±10.1 |
| Sex (no.) % | |
| Male | 24 (60) |
| Female | 16 (40) |
| CVRF, % | |
| Smoker | 43 |
| Hypertension | 55 |
| Dyslipidemia | 33 |
| Diabetes | 30 |
| BS | 1.9 (1.4-2.3) |
| ECG | |
| LBBB, % | 48 |
| Echocardiograph | |
| LVEDD, mm | 65±7 (56-79) |
| LVESD, mm | 55±9 (37-74) |
| LVEF, % | 28±8 (15-40) |
| Asynchrony, % | 43 |
| NYHA FC, no./No. (%) | |
| I | 3/40 (7) |
| II | 19/40 (48) |
| III | 15/40 (38) |
| IV | 3/40 (7) |
| Catheterization | |
| LCB, ADA prox., ≥2 vessels >75%, no./No. % | 14/40 (35) |
| CMR | |
| LVEDV, ml/m2 | 139 (100-255) |
| LVESV, ml/m2 | 103 (51-210) |
| LVEF, % | 27 (10-40) |
ADA, anterior descending artery; BS, body surface; CMR, cardiac magnetic resonance; CVRF, cardiovascular risk factors; ECG, electrocardiogram; FC, functional class; LBBB: left bundle branch block; LCB, left coronary branch; LVEDD, left ventricular end-diastolic diameter; LVEDV, left ventricular end-diastolic volume; LVEF, left ventricular ejection fraction; LVESD, left ventricular systolic diameter; LVESV, left ventricular end-systolic volume; NHYA, New York Heart Association.
The 40 patients completed all examinations and all MDCT studies were interpretable. In compliance with the Felker et al.17 coronary angiography criteria in invasive coronary angiography, 35% (14/40) were classified as ischemic LVSD.
Sensitivity and specificity values of 100% and 31%, respectively, were obtained for the presence of coronary calcium in the coronary LVSD identification. If an Agatston calcium score >100 is considered, specificity rises to 58% while maintaining 100% sensitivity.
The sensitivity and specificity of coronary angiography by MDCT for identifying coronary LVSD were 100% and 96%, respectively, while the results for the identification of necrosis areas by signal hypoattenuation in early acquisition were 57% and 100%, and 84% and 96% by LIE in the late acquisition.
Table 2 summarizes these results.
Table 2. Diagnostic Value of the Different Multidetector Computed Tomography Resources.
| TP | TN | FP | FN | Sensitivity | Specificity | PPV | NPV | |
| Coronary calcium | 14 | 8 | 18 | 0 | 100 (96-100) | 31 (11-50) | 44 (25-62) | 100 (94-100) |
| Calcium score >100 | 14 | 15 | 11 | 0 | 100 (96-100) | 58 (37-79) | 56 (35-77) | 100 (97-100) |
| Coronary angiography | 14 | 25 | 1 | 0 | 100 (96-100) | 96 (87-100) | 93 (77-100) | 100 (98-100) |
| Hypoattenuation | 8 | 26 | 0 | 6 | 57 (28-87) | 100 (98-100) | 100 (94-100) | 81 (66-96) |
| Late iodine enhancement | 12 | 25 | 1 | 2 | 86 (64-100) | 96 (87-100) | 92 (74-100) | 93 (81-100) |
FN, false negatives; FP, false positives; NPV, negative predictive value; PPV, positive predictive value; TN, true negatives; TP, true positives.
Diagnostic values are percentages, with 95% confidence interval values in brackets.
Comparing the groups established by MDCT (coronary angiography-MDCT and LIE-MDCT) with those determined by invasive coronary angiography-LGE-CMR (Table 3), 12 of the 13 patients in group 1 (κ 0.94, P<.001), 24 of the 25 patients in group 2 (κ 0.95, P<.001) and the only patient in group 3 were correctly identified. Those incorrectly classified were 2 of the 3 patients in group 4 (κ 0.48, P<.001). Therefore, all “ischemic” patients were correctly identified by MDCT: those meeting for coronary angiography criteria with necrosis and the only patient with necrosis who did not comply with coronary angiography criteria (what could be called an “unrecognized ischemic” patient). The MDCT misidentified 2 patients in group 4 (meeting coronary angiographic criteria but without necrosis). One of these cases met LVSD coronary angiography criteria but the necrosis was not identified by MDCT; the severity of the coronary lesions were overestimated in the second case due to intense calcification (Table 3). The sensitivity and specificity for identifying ischemic patients by noninvasive coronary angiography and with necrosis (group 1) was 92% (95% confidence interval [95%CI], 74%-100%) and 100% (96%CI, 88%-100%), shown in Figure 1. For all coronary patients with noninvasive coronary angiography with or without necrosis (groups 1 and 4), the results were 100% (95%CI, 96%-100%) and 96% (95%CI, 87%-100%), respectively. The only “unrecognized ischemic” patient, ie, the one without coronary lesions but with necrosis, was correctly identified by both techniques (Figure 2).
Table 3. Classification of Patients With Left Ventricular Dysfunction Based on Coronary Angiographic Data and the Detection of Necrosis.
| LVSD MDCT | LVSD Catheterization and CMR | ||||
| Group 1 | Group 2 | Group 3 | Group 4 | Total | |
| Group 1 | 12 | 0 | 0 | 0 | 12 |
| Group 2 | 0 | 24 | 0 | 0 | 24 |
| Group 3 | 0 | 0 | 1 | 0 | 1 |
| Group 4 | 1 * | 1 * | 0 | 1 | 3 |
| Total | 13 | 25 | 1 | 1 | 40 |
CMR, cardiac magnetic resonance; LVSD, left ventricular systolic dysfunction; MDCT, multidetector computed tomography.
Group 1: “ischemic” patients, ie, meeting coronary angiography criteria, with necrosis; Group 2: “nonischemic” patients, ie, not meeting coronary angiography criteria, without necrosis; Group 3: the only “nonischemic” patient with necrosis, correctly identified by both techniques; Group 4: “ischemic” patients without necrosis.
* False positives by MDCT.
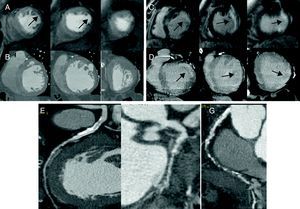
Figure 1. Group 1 patient with circumflex artery significant lesions (F) and right coronary artery occlusion (G) as well as calcified lesion, moderate in half anterior descending artery (E). First-pass perfusion defect (arrows) in cardiac magnetic resonance imaging (A) without areas of hypoattenuation detected on multidetector computed tomography (B). Late gadolinium enhancement in lateral segments (arrows) (C) and homologous late iodine enhancement in multidetector computed tomography.
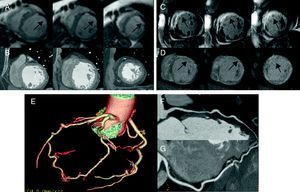
Figure 2. Group 3 patient without coronary lesions (E–G). First-pass perfusion defect (arrows) in cardiac magnetic resonance (A) without hypoattenuation being detected on multidetector computed tomography (B) and extensive necrosis (arrows) in cardiac magnetic resonance (C) and homologous multidetector computed tomography (D).
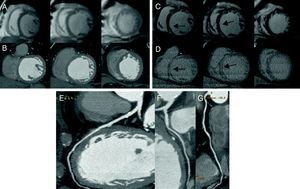
Six patients in group 2 had intramyocardial LGE suggestive of fibrosis and all were identified by LIE (Figure 3).
Figure 3. Group 2 patient without significant coronary lesions (E–G), without perfusion defects in cardiac magnetic resonance (A) nor areas of hypoattenuation in multidetector computed tomography (B) and with septal fibrosis (arrows) in cardiac magnetic resonance (C) and homologous multidetector computed tomography (D), but without necrosis.
The agreement between 2 experienced observers was analyzed, obtaining an excellent kappa value of 0.77 for identifying patients with LVSD of coronary etiology with necrosis (group 1) and of 0.89 in identifying patients with LVSD of coronary etiology with and without necrosis (groups 1 and 4).
DISCUSSIONThis is the first study in the literature to evaluate the diagnostic efficacy of all diagnostic tools available with MDCT to identify the coronary etiology of LVSD in the same group of patients.
In recent years, the diagnostic value of coronary calcium and coronary angiography have been published separately, and we have found only 1 study on the combination of coronary angiography and tissue characterization by LIE.
A study published more than 10 years ago evaluated the diagnostic potential of coronary calcium to identify LVSD of coronary origin, as this is a marker of coronary atherosclerosis.9 The authors report a sensitivity of 99% and specificity of 83%, increasing the latter in proportion to the score considered, reaching 100% if a cut-off point >220 was set for the Agatston score. A more recent study showed that a coronary calcium score of zero excludes the possibility of CHD to cause LVSD.10 Our study obtained a sensitivity of 100% (ie, all patients with ischemic LVSD had coronary calcifications) but with a very low specificity that improved somewhat with a cut-off calcium score >100, which identifies at least a moderate coronary atherosclerosis. This simple exploration that does not require contrast, with little radiation exposure, could be used to dismiss a cause of coronary origin in patients with ventricular dysfunction who show no coronary calcification, although its presence does not guarantee that coronary lesions will be found to explain LVSD.
Few studies have evaluated coronary angiography by MDCT in the etiological diagnosis of ventricular dysfunction, by comparing it with invasive coronary angiography in a patient and/or coronary segment analysis. These studies including a total of 357 patients with ventricular dysfunction show sensitivity values between 98% and 100% and specificity between 92% and 99%, similar to those in the population without LVSD.11, 12, 13, 14 Our study also obtained excellent diagnostic values (sensitivity 100% and specificity 96%), with the peculiarity that it ensures that coronary lesions detected justify the left ventricular dysfunction (meeting the Felker et al.17 criteria).
The use of LGE-CMR in the etiological study of ventricular dysfunction showed that there is a percentage of patients, between 10% and 15% depending on the studies, with necrosis and coronary artery disease without significant lesions in the catheterization.7, 8, 19, 20 These patients would be labeled as “nonischemic”, although based on the coronary angiography information they have a prognosis similar to that of “ischemic” patients who meet the Felker et al. coronary angiography criteria as demonstrated by our group.21 Experimental studies with computed tomography performed in the context of acute myocardial infarction have shown that iodine contrast has a similar kinetics to gadolinium, being able to identify affected areas as having signal hypoattenuation in the early acquisition with contrast or by late contrast enhancement in a late acquisition, usually 10min after administration.22 Human studies have confirmed the good correlation between magnetic resonance imaging and computed tomography to detect areas of infarction in both early and late acquisition.23, 24
Only 1 published study compared the combination of coronary angiography and the presence of necrosis by computed tomography in LVSD patients against invasive coronary angiography and LGE-CMR,14 obtaining excellent agreement as in our study. In that study's sample of 71 patients, there were 2 false negatives (one with fibrosis and the other with necrosis) attributed to poor study quality, and 3 false positives when detecting LIE with a necrosis pattern that was not confirmed by LGE-CMR, which the authors attributed to artifacts due to adjacent bone structures and/or movement. In our study, there was only 1 false negative in group 1, due to correctly identifying coronary lesions but not areas of necrosis, and 1 false positive in group 4 due to overestimating the severity of coronary lesions, which demonstrates the potential limitations of the MDCT. The first limitation, which is well known, is to overestimate coronary lesions in the presence of extensive calcification. The second is a technical limitation: although the kinetics of iodine is similar to that of gadolinium, it is not possible to cancel the signal from the myocardium with an inversion pulse as in CMR. This makes it difficult to identify necrosis areas, especially small ones. Interestingly, all areas of intramyocardial fibrosis were correctly detected by MDCT, possibly due to the good signal/noise ratio achieved when these areas become isolated from the intraventricular contrast septum.
LimitationsThe results of this study must be interpreted within the context that we used a small sample of selected patients, as use of the 64-detector scanner was limited in cases of higher heart rates and arrhythmias. Devices with a higher number of detectors are currently available, allowing interpretable studies in patients with high heart rates and even in atrial fibrillation. Improvements in detectors and dual-source computed tomography scanners also provide better tissue characterization.
CONCLUSIONSAll MDCT resources can be used to identify the ischemic etiology of LVSD, with coronary angiography-MDCT having the highest diagnostic accuracy when compared with catheterization. If the tissue information offered by MDCT is added, our results suggest that MDCT can be used as an alternative to the usual diagnostic approach of catheterization and RMC with contrast.
FUNDINGThis work was supported in part by a grant from the Fundación Española del Corazón and Fuente Liviana 2009 of Sociedad Española de Cardiología. F. Ridocci received assistance from the Instituto de Salud Carlos III Research Activity Intensification Programme 2010.
CONFLICT OF INTERESTNone declared.
Received 31 May 2011
Accepted 11 July 2011
Corresponding author: Unidad de Imagen Cardiaca, ERESA, Consorcio Hospital General Universitario de Valencia, Avda. Tres Cruces 2, 46014 Valencia, Spain. jestornell@eresa.com