Left bundle branch block (LBBB)-induced cardiomyopathy occurs in patients with long-standing LBBB. These patients characteristically exhibit hyperresponsiveness to cardiac resynchronization therapies (CRT). However, there is scarce information on their response to medical treatment. The aim of this study was to assess the change in left ventricular ejection fraction (LVEF) after a 3-month period following titration of guideline-directed medical therapy for heart failure.
MethodsThis retrospective analysis included all patients assessed in the heart failure unit of a Spanish University Hospital between 2020 and 2021, who presented with de novo ventricular dysfunction (LVEF <40%) and had a history of long-standing LBBB with no other possible causes of cardiomyopathy.
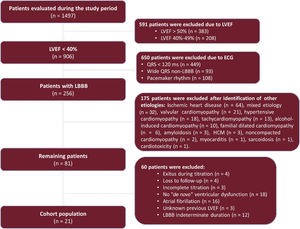
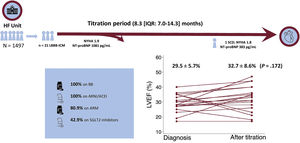
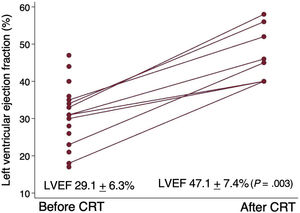
ResultsA total of 1497 patients were analyzed, of which 21 were finally eligible. Mean time from first diagnosis of LBBB to first consultation was 4.05± 4.1 years. Mean LVEF from first consultation to end of titration improved from 29.5±5.7% to 32.7±8.6% (P = .172), but none had recovered ventricular function at the end of follow-up. New York Heart Association functional class improved from 1.91±0.46 to 1.81±0.53 (P=.542). After CRT device implantation in 8 patients, LVEF improved by 18.1±6.4% (P=.003).
ConclusionsGuideline-directed medical therapy seems to be ineffective in improving LVEF and functional class in patients with de novo heart failure and LBBB-induced cardiomyopathy. Based on a positive response to CRT on LVEF improvement, early CRT implantation could be a reasonable strategy for these patients.
Keywords
Dilated cardiomyopathy is a heart disease consisting of dilatation of the left ventricular cavity, or of both ventricles, which is accompanied by systolic ventricular dysfunction and can be the consequence of various genetic predispositions and/or environmental noxae.1 The high prevalence and incidence of left bundle branch block (LBBB) in this group of patients (30% of cases),2 contrasts sharply with the 1% prevalence observed in the general population. Moreover, the prevalence increases progressively with age, reaching 3% in patients older than 80 years, often related to underlying structural heart disease.3,4 These observations reinforce the idea that LBBB could be an acquired disorder due to conduction disturbances that appear during the natural course of the disease. However, a small subgroup of patients with a long-standing history of LBBB and no evidence of structural heart disease at diagnosis have been described, who have progressive deterioration of ventricular function with marked intraventricular asynchrony during follow-up, and who are hyperresponsive to cardiac resynchronization therapies (CRT). This could suggest the existence of a distinct clinical entity in which electromechanical desynchrony caused by LBBB is the cause and not the consequence of the cardiomyopathy,5–8 known as LBBB-induced cardiomyopathy (LBBB-ICMP).
Current recommendations indicate that medical therapy is the cornerstone for HF patients with left ventricular ejection fraction (LVEF) <40% and suggest starting with a combination of beta-blockers, mineralocorticoid receptor antagonists, angiotensin-converting enzyme inhibitors (ACEI)/angiotensin receptor blockers (ARB) or angiotensin receptor-neprilysin inhibitors (ARNI). Recently, SGLT2 inhibitors have been added to this therapeutic strategy. However, there is little evidence of the effect of these drugs in patients with LBBB-ICMP, although it seems that their response would be worse than in patients with narrow QRS.9 In contrast, several retrospective studies have analyzed the response of patients with LBBB-ICMP to CRT and physiological pacing (His pacing or LBBB) in which, after correction of the asynchrony generated by LBBB,10 almost all patients improved their functional class and recovered normal LVEF, even with proven long-term prognostic benefits.5,11
In this context, the main objective of this study was to evaluate the effect on LVEF of new guideline-directed medical therapy (GDMT) for patients with HF and reduced LVEF in patients with LBBB-ICMP.
METHODSThis retrospective analysis included all patients with LBBB-ICMP who were admitted for an episode of HF and/or who were assessed for de novo ventricular dysfunction in the outpatient HF unit of the University Hospital of Santiago de Compostela between 2020 and 2021.
The inclusion criteria were: a) history of LBBB for at least 2 years with LVEF> 50% at diagnosis; b) sinus rhythm; c) LVEF <40% at first assessment prior to initiation of medical treatment; d) admission for HF or New York Heart Association functional class ≥ 2; and e) absence of other identifiable causes of cardiomyopathy, including severe mitral or aortic valve disease. The exclusion criteria were: a) underage patients; b) loss to follow-up during the process of drug titration; and c) loss of patient LVEF information at baseline or at the end of the study period.
Outpatient follow-up of patients after hospital discharge or after the first assessment consultation was done by dedicated personnel from the HF unit. Medical staff and specialized nursing staff were responsible for monitoring clinical events and titration of neurohormonal treatment in accordance with clinical practice guidelines until the maximum target doses of each drug or the maximum dose tolerated by the patient were reached.
The etiological study of the cardiomyopathy was carried out by specialist cardiologists and the tools used to complete the study included information from the clinical history, analytical parameters, electrocardiograms, transthoracic echocardiography, cardiac magnetic resonance imaging and coronary angiography, among others.
The electrocardiographic criteria used to diagnose LBBB were QRS duration> 130ms, absence of Q wave in leads I, V5 and V6, monomorphic R wave in I, V5 and V6 and ST-segment shift and T wave with opposite direction to the main deviation of the QRS complex12 and were reviewed one by one by an electrophysiologist (MRM).
LVEF was assessed at baseline and at the end of drug titration using Simpson's biplane method measured by transthoracic echocardiography or by cardiac magnetic resonance (CMR) in those patients with a suboptimal acoustic window for adequate quantification of ventricular volumes. This procedure is routinely performed in our institution at 3 months after the first medical contact to evaluate the referral for CRT/physiological pacing.
Information on personal history and comorbidities was obtained from the electronic medical records of each patient. The analytical parameters recorded were those obtained from the blood test performed for the first consultation.
All patients are part of a registry whose protocol has been approved by the ethics committee of our hospital. Informed consent was collected from patients participating in the study.
Guideline-directed medical therapyRecognized GDMT in the European Society of Cardiology guidelines for heart failure with reduced ejection fraction at the time of study performance were beta-blockers, ACEI/ARB/ARNI, and mineralocorticoid receptor antagonists±SGLT2 inhibitors. All GDMT assessments were made 12 months after patient enrollment into the registry. For each medication, initiation was defined as the start of treatment among patients who were not treated at enrollment. Dose escalation was defined as an increase to an average daily dose at least 10% higher than the average daily dose at enrollment among patients who were taking less than the target dose at enrollment or change from ACEI/ARB to ARNI. Achievement of the target dose was defined as an increase to a dose at or above daily guideline recommendations, among patients who were taking less than the target dose at enrollment. Patients were categorized as being on GDMT if they had documented drug use between echocardiograms for at least 90 days.
Statistical analysisCategorical variables are presented as percentages and continuous variables as mean±standard deviation or median [interquartile range]. Between-group comparisons were performed using the Pearson chi-square test and the Fisher exact test for categorical variables. For continuous variables, the Student t test and the nonparametric Mann-Whitney test and multivariate linear regression were used after determination of normal distribution with the Kolmogorov-Smirnov test. Statistical significance was set at P <.05. Data were stored in Microsoft Excel 2016 and statistical analyses and graphs were performed using Stata/IC version 16.1 software (StataCorp, United States).
RESULTSFrom January 2020 to December 2021, a total of 1497 patients were evaluated in the outpatient HF department. Of these, 854 patients had QRS <130ms, 314 LBBB, 162 pacemaker rhythm and 168 wide QRS non-LBBB. Only 21 patients with LBBB met the inclusion criteria for the study, of whom the initial event was an admission for HF in 10 (figure 1). The baseline and analytical characteristics, as well as the percentage of patients treated with each of the medications analyzed are shown in table 1.
Baseline characteristics of the study population
| Baseline characteristics | |
| Age, y | 67.9±13.0 |
| Female sex | 12 (57.1) |
| BMI | 29.9±3.7 |
| Hypertension | 13 (61.9) |
| Dyslipidemia | 10 (47.6) |
| Diabetes mellitus | 7 (33.3) |
| CKD | 5 (23.8) |
| Stroke/TIA | 3 (13.3) |
| COPD | 1 (4.8) |
| PAD | 1 (4.8) |
| Blood test variables | |
| Hemoglobin, g/dL | 14.0±1.2 |
| GFR, mL/min/1.73 m2 | 66.9±23.1 |
| Sodium, mEq/L | 139.6±2.2 |
| Potassium, mEq/L | 4.5±0.6 |
| Triglycerides, mg/dL | 97.3±58.3 |
| LDL, mg/dL | 96.2±39.7 |
| Albumin, g/dL | 4.3±0.4 |
| HbA1c | 5.9±0.8 |
| Medication after titration | |
| BB | 21 (100) |
| ARNI | 20 (95.2) |
| ACEI | 1 (4.8) |
| MRA | 17 (80.9) |
| SGLT2i | 9 (42.9) |
| Diuretics | 7 (33.3) |
ACEI, angiotensin-converting enzyme inhibitors; ARNI, angiotensin receptor-neprilysin inhibitors; BB, beta-blockers; BMI, body mass index; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; GFR, glomerular filtration rate; LDL, low-density lipoprotein; MRA, mineralocorticoid receptor antagonists; PAD, peripheral arterial disease; SGLT2i, SGLT2 inhibitors, TIA, transient ischemic attack.
Data are presented as absolute frequencies (relative frequency in %) or mean±standard deviation.
To complete the etiological study, at least 1 transthoracic echocardiogram was performed in all patients, while coronary angiography was performed in 4 patients, with no evidence of significant coronary lesions in any of them. CMR was performed in 19 patients, of whom 11 had no late gadolinium enhancement (LGE); 6 had LGE in the basal right ventricle insertion (nonspecific finding), and 1 had an intramyocardial septal pattern and in both right ventricle septal insertions.
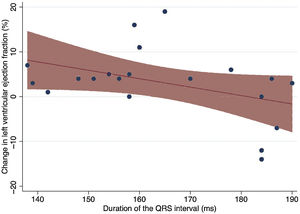
The mean time from first diagnosis of LBBB to first consultation was 4.05±4.1 years. The mean QRS duration at the first consultation was 163.7±17.2ms, with an axis of −3.6° and a mean QTc duration of 478.7ms. There was a trend toward a decreased response to pharmacological treatment in patients with a longer QRS, although this did not reach statistical significance (r=−0.4128; P=.07) (figure 2).
After a median follow-up time of 8.3 [7.0-14.3] months, there was a reduction in N-terminal pro-B-type natriuretic peptide levels from the first consultation from a median of 1081 [651.5-2619] pg/mL to 383 [169-1084] pg/mL (P=.027). However, this did not lead to a significant change in NYHA class (1.91±0.46 at baseline vs 1.81±0.53 at the end of titration; P=.542). The absolute improvement in LVEF was 3.12±2.27% (29.5±5.7% to 32.7%±8.5%; P=.172) and the mean reduction in left ventricular end-diastolic volume was 17.5±39.9mL (189.5±69.7mL to 172.0±43.7mL; P=.055). No patient achieved LVEF normalization (LVEF> 50%), 2 remained stable, and 3 patients showed worse LVEF (figure 3). A secondary multivariate analysis was conducted, including LVEF and end-diastolic volume at baseline follow-up, medical treatment, and duration of medical treatment; pretreatment LVEF and duration of pharmacological titration were not significantly associated with improvement in LVEF (−0.52% per 1% increase in pretreatment LVEF (95% confidence interval, −1.64 to +0.60; P=.323) and −0.41% for each month of follow-up (95% confidence interval, −1.90 to +1.08; P=.548)).
Central Illustration. Change in patients’ LVEF after pharmacologic titration to guideline-directed medical therapy and percentage of patients treated with each drug. ACEI, angiotensin-converting enzyme inhibitors; ARNI, angiotensin receptor-neprilysin inhibitors; BB, beta-blockers; HF, heart failure; IQR, interquartile range; LBBB-ICMP, left bundle branch block-induced cardiomyopathy; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonists; NYHA, New York Heart Association; SCD, sudden cardiac death.
During follow-up, 2 patients were admitted to hospital for decompensated HF. One patient was admitted during the titration period and the second several months after the end of this period in the setting of atrial fibrillation with rapid ventricular response and was referred for catheter ablation.
After pharmacological titration, 7 patients (33.3%) were implanted with a CRT-defibrillator device and 1 patient (4.8%) underwent CRT device implantation. All procedures were successful and there were no complications during device implantation. Only 1 patient developed pacemaker wound infection in the months following implantation. In the remaining patients, 2 refused device implantation, 3 were pending decision, 3 were not candidates due to comorbidities, 3 did not meet the criteria for CRT due to LVEF> 40%, 1 did not meet the criteria after improvement to NYHA functional class I, and 1 patient died suddenly before the decision was made.
After a median of 8.7 [5.9-9.4] months after CRT device implantation, the observed improvement in mean LVEF in the device implanted group was 18.1±6.4% (P=.003) (figure 4) and the reduction in end-diastolic volume was 37.4±23.7mL (P=.003).
DISCUSSIONOur study highlights the likely poor response to optimal GDMT among patients with de novo ventricular dysfunction secondary to asynchrony produced by long-standing advanced LBBB. No significant improvement in functional class, end-diastolic volume or LVEF was observed in patients after pharmacological titration. In fact, no patient recovered LVEF after pharmacological titration and 3 patients experienced worsening of LVEF at the end of the process. Importantly, sudden cardiac death occurred in 1 patient during the titration period. On the other hand, there was a significant improvement in LVEF and reduction in end-diastolic volume in patients who were redirected to CRT.
BackgroundOne of the major issues of this study was the difficulty identifying patients with LBBB-ICMP. This entity is not formally included among unclassified cardiomyopathies or among the acquired/nongenetic forms of dilated cardiomyopathy and with different criteria depending on the definition. Consequently, it is usually a diagnosis of exclusion after ruling out other possible etiologies and after assessing the patient's response to CRT. In recent years, Sanna et al.13 proposed diagnostic criteria based on clinical, genetic, echocardiographic and CMR parameters that distinguish the characteristics of patients with LBBB-ICMP. In our study, only 1 of the 19 patients who underwent CMR to complete the etiological study had an intramyocardial septal LGE pattern; the others had no LGE. CMR plays a key role in patients with dilated cardiomyopathy, not only to identify some etiologies with characteristic LGE patterns, but also to identify patients who may be more responsive to CRT and who are at higher risk of sudden cardiac death. Those with a higher lateral/septal diastolic thickness ratio and lateral wall thickening may have a greater response to CRT and these could be indicators of the temporal sequence of LBBB and cardiomyopathy.14
LBBB-ICMP was first described more than a decade ago, following the publication of the first trials with CRT therapies in HF patients. A subgroup of patients with nonischemic dilated cardiomyopathy and LBBB were found to be hyperresponders to CRT, achieving full functional recovery associated with normalization of LVEF. These results suggested that asynchrony caused by LBBB could be the etiological cause of cardiomyopathy.6,11 Vernooy et al.15 observed a worsening of LVEF, an increase in left ventricular cavity volume and an increase in left ventricular lateral wall mass in an 8-dog animal model to assess the effects of LBBB on cardiac function. The pathophysiological mechanism is based on conduction block in the Purkinje system, which causes mechanical dyssynchrony due to earlier contraction of the right ventricle free wall and interventricular septum relative to the LV lateral wall. This dyssynchrony and the imbalance of intraventricular pressures lead to dysfunction of the septal microvasculature and affect not only systolic function, with an increase in LV volume and a decrease in LVEF, but also LV diastolic function, causing a shortening of ventricular filling and thus contributing to cardiac remodeling.16,17
Over the past 2 decades, several clinical trials have been published demonstrating the benefits of CRT in terms of reduced HF hospitalizations and mortality in patients with LVEF <35% and LBBB> 130ms.18–20 Indeed, the latest clinical guidelines include the implantation of a CRT device with level of evidence IA in symptomatic patients with HF in sinus rhythm with LVEF <35%, QRS duration> 150ms and LBBB QRS morphology despite optimal medical therapy to improve symptoms and reduce morbidity and mortality.8,21 Regarding the optimal time to indicate CRT/physiological pacing, the NEOLITH study evaluated the LVEF response to GDMT at 3 months and early CRT in LBBB associated with idiopathic nonischemic dilated cardiomyopathy. The improvement in LVEF after GDMT in the LBBB group was 3.3%±10.7%, with a superresponse to CRT in 35% of LBBB participants, defined as a post-CRT LVEF ≥ 50%.22 In a retrospective cohort of 123 patients with new-onset LBBB-ICMP who underwent CRT implantation, the NEOLITH II trial compared the differences in clinical and echocardiographic prognosis as a function of time from diagnosis to CRT (≤ 9 months vs> 9 months). Clinical outcomes were similar in the 2 groups, but improvement in LVEF to> 35% was more likely in those implanted ≤ 9 months.23 In the subset of patients with LBBB-ICMP, Vaillant et al.5 demonstrated in 6 patients that who CRT implantation resulted in a significant improvement of NYHA functional class, normalization of left ventricle dimensions, near disappearance of mechanical dyssynchrony and LVEF improvement (from 31±12% to 56±8%).Physiological pacing therapies have also been shown to be effective in improving LVEF in patients with LBBB-ICMP and could represent an alternative to conventional CRT.24,25 However, GDMT, not CRT, is the first-line therapy for patients with reduced LVEF with LBBB, even though there are few data on how patients with reduced LVEF and LBBB respond to GDMT. Current guidelines recommend at least 3 months of GDMT prior to implantation of CRT, in the hope that medical therapy alone will improve LVEF. Nevertheless, it is worth emphasizing that none of the major trials supporting medical therapy stratified outcome analyses by the presence or absence of LBBB or reported QRS morphology as a baseline clinical characteristic.26–36 Importantly, there are no data regarding ARNI and LBB-ICM. Although based on a small number of patients, in our population, 95.7% patients were under ARNI without marked differences in terms of LVEF improvement., In contrast, a significant proportion of patients were not treated with iSGLT2, due to changing recommendations in clinical practice guidelines published during the study period.
Clinical relevanceLBBB-ICMP is a rare disease37 and is probably underdiagnosed. Its diagnosis is most often established retrospectively and after exclusion of other possible causes of cardiomyopathy. The low prevalence and lack of established criteria for early diagnosis hamper the performance of prospective studies analyzing the response to different therapeutic strategies in a controlled manner and with a sufficient sample size. However, with the results of the present study and the existing evidence of excellent response to CRT with short- and long-term clinical and prognostic benefits, it is possible that patients with LBBB-ICMP may benefit from an early strategy with CRT or LBBB pacing, as these are the only therapies that have so far demonstrated clinically important improvement in this patient profile. Delaying the implantation of these devices could mean a significant loss of time in stopping disease progression. Importantly, as in our sample, there is the potential risk of sudden cardiac death (1 out of 21).
LimitationsThis study has some limitations. First, this is a retrospective observational study without a control group in which a single-arm pre-post analysis was performed, with all the possible biases inherent to this type of design. In addition, to allow us to analyze response to pharmacological treatment and be able to prospectively include patients, response to CRT was not included as a criterion for LBBB-ICMP, and therefore patient characteristics in this study could differ slightly from those of other studies analyzing this condition. A genetic study could only be performed in a small proportion of patients. Empagliflozin and dapagliflozin are now recommended for the treatment of chronic heart failure with reduced ejection fraction, according to the latest 5-year update of the European Society of Cardiology. In our study, several patients completed the titration period before the publication of these guidelines, which is why only 43% of them were receiving SGLT2 inhibitors. Although this constitutes a limitation of the present study, the remaining key pillars of HF treatment were highly optimized, with 100% of our patients receiving BB, 95% ARNI and 80% receiving mineralocorticoid receptor antagonists at the maximal tolerated dose. At our center, as per protocol, the time interval between the implantation of the device and the performance of the echocardiogram is 6 months, whereas the titration period is 3 months. Therefore, there is a 3-month difference between one period and the other that may have led to overestimation of the response to CRT over GDMT. Although LBBB-induced cardiomyopathy is a rare condition, the small sample size does not allow definitive conclusions to be drawn about the effects of medical treatment and CRT in this group of patients. Further prospective and randomized studies with a larger sample size will be necessary to confirm the hypotheses generated.
CONCLUSIONSGDMT including BB, mineralocorticoid receptor antagonists, ACEI/angiotensin receptor blocker/ARNI±SGLT2 inhibitors seems to be ineffective in improving LVEF and functional class in patients with de novo HF and LBBB-ICMP. CRT may perform better in improving and recovering LVEF than GDMT in patients with LBBB-ICMP, and therefore consideration of early CRT implantation could be a reasonable strategy in this subset of patients.
FUNDINGNone.
AUTHORS’ CONTRIBUTIONSAll authors included in the study have been involved in the design, collection or analysis and interpretation of the data, writing of the text or final revision of the presented text.
CONFLICTS OF INTERESTThis research has not received any specific grant from any public, commercial, or not-for-profit sector. The authors declare that there is no conflict of interest.
- •
Left bundle branch block-induced cardiomyopathy is a rare condition characterized by hyperresponsiveness to cardiac resynchronization therapy. However, there is little evidence regarding the effect of medical treatment in this patient group, despite being the first step in the treatment of all patients with heart failure with LVEF <40%.
- •
This is one of the first studies to analyze and demonstrate the hypothesis that the new guideline-directed medical therapy with the new pharmacological groups for heart failure would not be effective in improving LVEF or functional class in patients with LBBB-ICMP as they are unable to correct the intraventricular asynchrony generated by LBBB.