Left bundle branch block (LBBB) is a very common finding in patients with heart failure (HF), particularly in the context of dilated cardiomyopathy, with publications showing its presence in up to 31% of dilated cardiomyopathy patients at their initial diagnosis.1 This figure can be even higher, depending on when the relevant study is conducted during the disease course. LBBB has traditionally been considered a consequence of the underlying cardiomyopathy,2,3 and not its cause, and it has been associated with a worsening of the prognosis of the affected patients.
The cardiomyopathy induced by LBBB is a hard-to-define and -demonstrate entity that is caused by the LBBB-induced electromechanical asynchrony and is not a consequence of it. The abnormal depolarization gives rise to mechanical asynchrony, with unusual septal motion and an apical rocking associated with a delayed contraction of the posterolateral segments. This asynchrony affects not only systolic function, but also diastolic function, with a shortening of the left ventricular (LV) filling time, which eventually leads to ventricular remodeling with elevated volumes and a lower LV ejection fraction (LVEF).4 It seems understandable, at least from a pathophysiological viewpoint, that correction of the conduction disorder via cardiac resynchronization therapy (CRT) or physiological conduction system pacing beyond the blockage point would lead to recovery of the LVEF, as long as there is no underlying structural or ultrastructural damage. However, the marked response to CRT with complete normalization of the LVEF in some patients (“hyperresponders”) actually suggested the existence of this entity more than a decade ago5,6 and spurred attempts to identify and distinguish it from other types of cardiomyopathy.7,8 This hyperresponse to CRT that characterizes LBBB-induced cardiomyopathy has also led to analysis of the potential use of left bunch branch pacing to improve the LVEF and functional class, with evidence also of LVEF normalization and functional class improvement with physiological pacing.9
The early detection of LBBB-induced cardiomyopathy may have major clinical and therapeutic implications, with a potential early benefit from CRT, a better clinical course, and a much more favorable prognosis than in conditions caused by a primary alteration of myocytes. Reliable measures are required to enable an early diagnosis of LBBB-induced cardiomyopathy with sufficient diagnostic certainty, instead of its diagnosis when the LVEF normalizes after correction of the conduction disorder. For this early detection, Sanna et al.10 proposed the combined use of various parameters that would act as “red flags”: parameters from ECG (typical LBBB pattern), echocardiography (normal thicknesses, without major chamber dilatation or global hypokinesia), and magnetic resonance imaging (without significant fibrosis or scarring), as well as the absence of family/genetic history and exclusion of other potential causes. However, its diagnosis is still based on exclusion and certainty is retrospectively obtained. The publication by Sanna et al. already recommended the early use of CRT. This is similar to the proposal of Wang et al.11 in the NEOLITH II study, in which the use of CRT in the first 9 months after the diagnosis of ventricular dysfunction was associated with beneficial cardiac remodeling and higher probability of LVEF recovery > 35%, although not with clinical or mortality endpoints.
In recent work performed in a Spanish referral center published in Rev Esp Cardiol, García-Rodeja Arias et al.12 retrospectively analyzed a total of 1497 patients admitted for HF or evaluated in the HF unit due to de novo LV dysfunction over a 2-year period. These authors ultimately obtained 21 eligible patients with LV dysfunction (LVEF < 40%) and in sinus rhythm with long-standing LBBB (duration of at least 2 years and with LVEF > 50% at diagnosis), with no identifiable causes of the cardiomyopathy. This already signals the rarity of this etiology, with just 1.4% of patients eligible from a tertiary referral hospital, as well as the authors’ rigorous selection of the patients to be included in the registry.
The time from first LBBB diagnosis to the first assessment in the HF unit was slightly more than 4 years on average. The patients’ drug therapy was highly optimized (100% with beta-blockers, 95.2% with angiotensin receptor-neprilysin inhibitors, 80.9% with aldosterone antagonists, and just 42.9% with sodium-glucose cotransporter type 2 [SGLT2] inhibitors); the latter is explained by the time at which the registry was begun, before publication of the major clinical trials and guidelines that have since established SGLT2 inhibitors as the first-line therapy. The LVEF improvement from the first visit to the end of the medical therapy optimization was 3.2 percentage points (from 29.5% to 32.7%; P=.172) and no patient had fully recovered LVEF at the end of follow-up. In addition, no improvements were found in functional class or LV end-systolic volume. However, in the same population with optimal medical therapy and with no LVEF improvement, implantation of a CRT device in 8 patients led to a significant improvement in the LVEF of 18.1%±6.4% and a reduction in the end-systolic volume by more than 37mL.
The authors conclude that optimal medical therapy according to clinical guidelines does not appear to effectively improve LVEF and functional class in patients with de novo HF and LBBB-induced cardiomyopathy but that a positive response to CRT should suggest early implantation. This conclusion is in line with those of previous work.13–15 Those studies also showed a low tendency for LVEF improvement after medical therapy optimization in patients with dilated cardiomyopathy and LBBB and also advocated for early CRT implantation.
The limitations of the work include those associated with a retrospective observational study with just 21 patients (1 of every 71 analyzed) and no control group, as well as the difficult definition of LBBB-induced cardiomyopathy: which came first, the chicken or the egg? Namely, does LBBB induce cardiomyopathy (dyssynchronization) or is LBBB onset the first manifestation in the natural course of dilated cardiomyopathy?16 The authors themselves mention the lack of homogeneity in the definition and classification of cardiomyopathy, whose diagnosis is typically based on exclusion. Only 8 of the 21 patients underwent CRT implantation for different reasons, and the outcomes could have been even more impressive and conclusive if this number had been higher. It is possible that a higher percentage of SGLT2 inhibitor use would have influenced the results (only 43% of patients were prescribed these drugs), although it seems unlikely based on the currently available evidence. Finally, coronary angiography was performed in only 4 patients (19%), which complicates our ability to rule out with absolute certainty the potential role of silent ischemia, despite examination of most of these patients with cardiac magnetic resonance imaging.
Although we have already commented on the difficult definitive diagnosis of cardiomyopathy with LBBB, it is important to suspect it at early stages in patients with known long-standing LBBB who develop HF during follow-up. Ponnusamy et al.9 used the following definition: a) LBBB identified more than 1 year ago, defined according to Strauss criteria; b) LVEF > 50% at LBBB diagnosis; c) progressive deterioration of the LVEF and functional class; d) no other identifiable cause of the cardiomyopathy; and e) echocardiographic evidence of dyssynchrony. It would be attractive to a priori consider early CRT implantation in these patients, but it must be remembered that no randomized controlled study has thus far shown that a few extra months of CRT implantation improve prognosis. It seems wise to continue to follow the guideline recommendations: first medical therapy optimization before consideration of CRT (if indicated) as soon as possible, even for ventricular dysfunctions that are not particularly severe but are clearly caused by LBBB (true dyssynchronopathy), and not years after, when the “moment” may have been lost for reverse remodeling if the initial electromechanical changes have already become irreversible structural changes. Physiological pacing, so much in fashion in recent years, likely adds weight to the evidence favoring the early treatment of LBBB-induced cardiomyopathy.
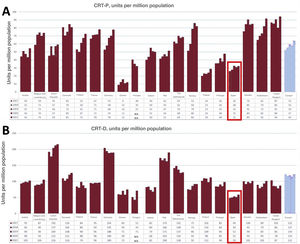
The low rate of CRT implantation in Spain remains worthy of attention. According to the Spanish Pacemaker Registry,17 3850 CRT devices were implanted in Spain in 2020 in total, comprising 1463 cardiac resynchronization therapy without defibrillation (CRT-P) devices and 2387 cardiac resynchronization therapy with defibrillation (CRT-D) devices. According to Eucomed data18 (figure 1), the CRT-D rate in Spain in 2021 was 59 units/million population while that of CRT-P devices was 32 units/million. However, that same year, the European averages were 122 CRT-D units/million and 64 CRT-P units/million, which shows that we continue to have at least half the average European rate of CRT implants.
CRT-P (A) and CRT-D (B) implantation rates per million population in different European countries and the European average (set of bars on the right) from 2017 to 2021. Highlighted with a box, data from Spain. Data reproduced with permission from MedTech Europe,18 based on reports from the main manufacturers.
We must thank the authors for their contribution to improving our understanding and approach to LBBB-induced cardiomyopathy. Their proposal for an early CRT-based management without waiting for medical therapy optimization, which goes beyond the current clinical practice guideline recommendations, requires a better definition of this cardiomyopathy from the first documentation of the ventricular dysfunction, as well as prospective studies with a significantly larger size that permit clear establishment of not only the response to CRT, but also the temporal relationship of this response to the LBBB onset. Is there a time limit for reversing the electromechanical changes induced by LBBB? Can a delay in the initiation of CRT miss a critical moment for reversing the progressive myocardial damage? Would cardiac magnetic resonance imaging enable the detection of patients with LBBB and potential associated myopathy who would benefit from early CRT? These and other questions must be answered using larger randomized studies before the systematic recommendation of early CRT implantation or physiological pacing.
FUNDINGNone.
CONFLICTS OF INTERESTFees for consultancy work from Medtronic and Abbott; honoraria for conferences, educational events, and presentations from Medtronic, Boston Scientific, and Bayer; conference attendance support from Daiichi Sankyo and Abbott; none of which were related to this article.