When we need to decide the best antiplatelet regimen for a patient who is to undergo percutaneous coronary intervention (PCI), multiple variables must be considered in an attempt to improve prognosis by reducing ischemic complications, without an unacceptable increase in bleeding events.
The aim of antiplatelet therapy in patients undergoing PCI is 3-fold: to reduce the thrombus burden and risk of no reflow in acute patients (in a clearly unstable setting), to reduce any damage from angioplasty (plaque rupture, iatrogenic dissection, occlusion of lateral branches, distal embolization), and, lastly, to prevent stent thrombosis, a complication that is becoming less common with modern stents, but which is highly relevant for prognosis.1
Therefore, the clinical features that determine revascularization constitute a key factor in deciding the intensity and duration of antiplatelet therapy. Patients with chronic syndromes (stable plaques without a thrombotic component or plaque rupture) are “less demanding” of potent antiplatelet drugs and usually require shorter dual antiplatelet therapy (DAPT) than patients revascularized for acute coronary syndrome (ACS).2,3 Patients with ACS are the main beneficiaries of prasugrel and ticagrelor, as well as antiplatelet regimens lasting longer than 1 year.3,4
However, it is becoming increasingly clear that clinical presentation is not everything and that we must consider the extent of coronary disease and the complexity of the PCI performed.
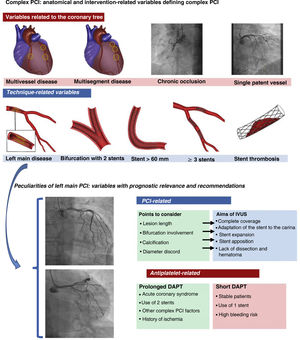
ANTIPLATELET THERAPY IN COMPLEX LESIONSThere is a gap in the evidence on the optimal duration of DAPT for patients undergoing complex PCI, whether due to the extent of disease or the complexity of the technique. The category of complex lesions encompasses the treatment of 3 or more lesions, implantation of 3 or more stents, long lesions requiring a stent> 60 mm, bifurcations treated with 2 stents, chronic occlusions, and left main disease (figure 1). We know that these complex lesions are associated with worse prognosis, particularly in the first year, due to a higher rate of ischemic events.5 Some studies indicate that prolonged DAPT improves prognosis in these patients by reducing ischemic events without a significant increase in bleeding complications; this benefit of prolonged DAPT is found particularly in patients with a DAPT score ≥ 2 (greater reduction in infarction and major ischemic events than patients on 12 months’ DAPT).5
The upper section shows the anatomical and technique-related variables that constitute complex PCI. The lower section shows important aspects of left main PCI. The anatomical variables that can be optimized with the use of IVUS in left main PCI are described, as well as the clinical and technical aspects that can influence the decision to prolong or shorten DAPT duration. DAPT, dual antiplatelet therapy; IVUS, intravascular ultrasound; PCI, percutaneous coronary intervention.
One aspect that is never discussed but which is real is that complex lesions, as well as having a higher intrinsic ischemic risk, can be associated with not-so-good intervention outcomes. In these lesions, stent underexpansion, malapposition, incomplete coverage of plaques, etc, can occur more often than in favorable lesions and are, undoubtedly, variables associated with worse ischemic prognosis. These suboptimal PCI outcomes are often not reflected in clinical reports, possibly because they are unknown. Certainly, one of the main limitations of angiography is the assessment of the PCI result in these types of lesions.
Returning to the subject of antiplatelet therapy, the latest European guidelines on non–ST-elevation acute myocardial infarction3 go further, giving prominence to the recommendation (class IIa A indication) for prolonged DAPT in patients with high risk of ischemic events, based on clinical judgement, medical history, and coronary anatomy. The authors encourage us to take into consideration a series of clinical (diabetes mellitus, recurrent infarction, multivessel disease, polyvascular disease, and moderate renal impairment) and angiographic (described above) risk criteria. The current position in these guidelines is the recommendation that these patients receive prolonged DAPT provided they are not at high risk of bleeding.
Therefore, it is no longer just the clinical presentation that will determine which patients would benefit most from prolonged DAPT, but also the angiographic features and the percutaneous technique.
The new antiplatelet agents should always be the drugs of choice in ACS if there is no contraindication, and in prolonged DAPT regimens if the risk of bleeding is not high.3 However, just as we were getting some clarity on this subject, it is further complicated by the findings in some recent studies in this population, in which monotherapy with potent antiplatelet agents gave lower rates of major bleeding than DAPT, without an increase in ischemic events. Data from the Twilight trial in patients undergoing complex PCI showed that patients on ticagrelor monotherapy from 3 months, compared with those on ticagrelor plus aspirin for 12 months, had a lower incidence of major bleeding (1.1% versus 2.6%; hazard ratio [HR],0.41; 95% confidence interval [95% CI], 0.21-0.80), with no difference in ischemic events (death, infarction or stroke) (3.8% vs 4.9%; HR, 0.77; 95%CI, 0.52-1.15) or stent thrombosis.6 Of note, only 30% of these patients had revascularization due to acute myocardial infarction, and the Twilight trial contained mostly stable patients. Therefore, in this type of lesion, ticagrelor monotherapy appears to be as effective as DAPT, but with better safety.
ANATOMICAL PECULIARITIES AND LEFT MAIN PCI TECHNIQUESLeft main coronary artery disease is present in 5% of catheterization procedures performed in patients with ischemia. In Spain, around 4000 patients with left main disease are treated with PCI each year.7
The approach must be meticulous, due to the potential effect of complications during and after the procedure and the prognostic implication of a good result. Undoubtedly, modern stents have reduced acute and late thrombosis and are an indispensable tool that has greatly contributed to improved prognosis. However, there are other important aspects that should be taken into account when performing PCI of the left main: this segment always involves large diameter vessels, considerable discord between proximal and distal segments, and a very high percentage of patients require treatment of a bifurcation—the largest one in the coronary tree. The potential technical problems are clear: risk of stent underexpansion or malapposition, and the presence of 2 or 3 layers of stent at the level of the carina (when the treatment of the left main involves 2 stents), aspects that have been associated with higher incidence of ischemic events when DAPT is discontinued.8
Therefore, it is essential to ensure an optimal result, which ultimately will be associated with better prognosis. Intracoronary imaging, with intravascular ultrasound (IVUS) or optical computed tomography, is one of the fundamental pillars that can help achieve this (figure 1). These techniques provide important diagnostic information (diameter, calcification, lesion eccentricity, involvement of the coronary ostia of the left anterior descending and the circumflex artery) that will help us to design the best therapeutic strategy. In addition, they compensate for the major limitations of conventional angiography when evaluating PCI results and allow diagnosis of stent underexpansion or malapposition, which are very common in such lesions. Optimization of left main PCI, essentially with IVUS guidance, has demonstrated improved prognosis in these patients vs PCI performed with angiography alone,9 to the extent that the most recent European guidelines on revascularization recommend it with a class IIa indication.10
SHOULD WE EXTEND ANTIPLATELET THERAPY IN LEFT MAIN PCI?For the reasons above (atherosclerotic disease in the most important segment of the coronary tree, the technical characteristics of the PCI, and the risk of stent thrombosis, which still occurs in 0.7% to 3% at 3-5 years of follow-up11,12), the question always arises of which and how much antiplatelet therapy to use.
Obviously left main disease will always be included in the category of complex lesions, but despite the importance of the problem, there is still a lack of relevant evidence to guide the decision to use one antiplatelet regimen over another. This is, firstly, because these patients are under-represented in the area of complex lesions and, secondly, because the research on left main PCI has generally been performed to demonstrate its efficacy and safety in comparison with surgery, which until a few years ago was almost the only revascularization strategy for these patients.11,12
Therefore, the evidence for or against prolonged DAPT regimens in left main PCI is limited and often discordant. Such discordance is largely explained by the small number of patients analyzed and use of post-hoc analyses in studies designed with other objectives.
In light of these issues, the recently published findings by Cho et al.13 in Revista Española de Cardiología should be considered of great value. The authors analyzed, in 1827 patients from 2 prospective multicenter registries who underwent left main stem PCI with second-generation stents, the prognostic effect of prolonging DAPT. Prolongation of DAPT (> 12 months) was associated with a lower incidence of ischemic events. Specifically, the HR adjusted for ischemic events (cardiac death, infarction, or stent thrombosis) was significantly higher in patients with DAPT <6 months (HR,4.51; 95%CI, 2.96-6.88) and DAPT for 6 to 12 months (HR,1.92; 95% CI, 1.23-3.00) than in those with DAPT extended beyond 1 year. This significant clinical benefit was not associated with more bleeding events in the group with prolonged DAPT, who showed a particularly low incidence. This similar, low incidence of bleeding in the mid-term in patients with left main PCI has also been reported by other groups.14
The good outcomes published in the article by Cho et al.13 prompt several comments. First, the group has extensive experience in left main PCI (as demonstrated by the high number of patients treated, as well as the prominent articles published in this field), a point that should always be taken into consideration. In this study, 75.4% of the patients were treated due to ACS. Regarding the technique, the strategy of choice was angioplasty with 1 stent (83.3%) and IVUS was used in 60.7%. These are very important points and should be considered in any attempt to reproduce the results.
In addition, the low incidence of bleeding events can be explained by aspects that perhaps involve more bias: use of the Thrombolysis in Myocardial Infarction (TIMI) definition of bleeding; a very low usage of new antiplatelet agents (5.4%) in a population with mostly ACS and multivessel disease in 70.1%; and prolonged DAPT was prescribed in patients without a history of hemorrhagic events, which is therefore a selected population. However, this last point should not be considered a limitation, but rather more of a strategy. As the authors discussed, their findings should be interpreted as provisional and hypothesis-generating and, of course, should be confirmed in clinical trials designed to answer this question.
To summarize and conclude, it is true that the indication for DAPT is determined primarily by the clinical presentation but, with the information available and the recommendations from the clinical guidelines, perhaps we should consider prolongation of DAPT in cases of complex lesions or left main PCI provided that the patient has not had bleeding events at 6 or 12 months. These strategies will reduce ischemic events in patients with higher risk, probably with low costs.
However, it is undeniable that we need more relevant information: definitive studies that analyze this question in this specific, complex population, and that ultimately reaffirm the current recommendations. Until this information is available, the antiplatelet regimen should be individualized for each patient. In addition to the clinical presentation and risk of ischemia and bleeding, we must also consider the coronary anatomy, the extent of disease, and the complexity of the PCI. All of this should guide us to recommend the optimal antiplatelet regimen for each individual patient. In addition, we must not forget that these recommendations should not be static but should be adapted to any clinical circumstances that may arise.
FUNDINGNone.
CONFLICT OF INTERESTSJ.M. Ruiz-Nodar declares having received honoraria for presentations from AstraZeneca, Biosensor, and Terumo.
ACKNOWLEDGEMENTSWe wish to thank Marta Terol Ballester for the illustrations in this article.