The management of persistent moderate-severe tricuspid regurgitation (TR) in patients with chronic thromboembolic pulmonary hypertension after treatment with pulmonary endarterectomy (PEA) or balloon pulmonary angioplasty (BPA) is not well defined. This study aimed to analyze the progression and predictors of significant persistent postintervention TR and its prognostic impact.
MethodsThis single-center observational study included 72 patients undergoing PEA and 20 who completed a BPA program with a previous diagnosis of chronic thromboembolic pulmonary hypertension and moderate-to-severe TR.
ResultsThe postintervention prevalence of moderate-to-severe TR was 29%, with no difference between the PEA- or BPA-treated groups (30.6% vs 25% P=.78). Compared with patients with absent-mild postprocedure TR, those with persistent TR had higher mean pulmonary arterial pressure (40.2±1.9 vs 28.5±1.3mmHg P <.001), pulmonary vascular resistance (472 [347-710] vs 282 [196-408] dyn.s/cm5; P <.001), and right atrial area (23.0 [21-31] vs 16.0 [14.0-20.0] P <.001). The variables independently associated with persistent TR were pulmonary vascular resistance> 400 dyn.s/cm5 and postprocedure right atrial area> 22cm2. No preintervention predictors were identified. The variables associated with increased 3-year mortality were residual TR and mean pulmonary arterial pressure> 30mmHg.
ConclusionsResidual moderate-to-severe TR following PEA-PBA was associated with persistently high afterload and unfavorable postintervention right chamber remodeling. Moderate-to-severe TR and residual pulmonary hypertension were associated with a worse 3-year prognosis.
Keywords
In recent years, the treatment of tricuspid regurgitation (TR) has undergone changes, prompted by the negative impact of this condition on mortality and morbidity1,2 and the introduction of alternatives to surgery, mostly percutaneous techniques.3 TR is functional in more than 90% of patients and is closely associated with left heart disease and precapillary and postcapillary pulmonary hypertension (PH).4 Experience in the treatment of this condition mainly comes from patients who have undergone surgery for mitral valve disease. In these patients, TR often persists after surgery or worsens during follow-up, prompting the recommendation of tricuspid valve repair during the index intervention.5 Treatment is less well established in isolated TR. In TR associated with PH or right ventricular dysfunction, clinical practice guidelines do not recommend tricuspid valve intervention.6,7
The treatment of choice in patients with operable chronic thromboembolic pulmonary hypertension (CTEPH) is pulmonary endarterectomy (PEA).8 This interventional procedure is highly effective, producing excellent hemodynamic outcomes and reducing symptoms and mortality. However, up to 40% of patients with CTEPH are inoperable, due either to lesion inaccessibility or to the presence of comorbidities.9 In these patients, balloon pulmonary angioplasty (BPA) is a safe and effective alternative that yields satisfactory hemodynamic, clinical, and functional outcomes.10
TR in patients with CTEPH undergoing PEA or BPA is usually treated successfully with a conservative approach, and the severity of TR decreases after successful surgery.11,12 However, in some patients, TR persists after the intervention, and information is lacking on the clinical, hemodynamic, and echocardiographic characteristics of these patients and how persistent postintervention TR impacts prognosis during follow-up, especially in patients treated with BPA. It is also unknown whether some of these patients would benefit from subsequent surgical or percutaneous tricuspid valve intervention.
The aim of this study was to analyze the status of moderate-to-severe TR in patients with CTEPH treated by PEA or BPA and to identify predictors of persistent TR after surgery and its impact at 3-year follow-up.
METHODSPatient selectionThis retrospective observational study included consecutive patients with CTEPH who underwent PEA or completed a BPA program between January 2010 and October 2021 at a Spanish national center with specialist expertise in PH. In all patients, the diagnosis of CTEPH was confirmed according to the pre-2022 diagnostic and hemodyamic criteria for precapillary PH (mean pulmonary arterial pressure ≥ 25mmHg, pulmonary vascular resistance ≥ 240dyns/cm5 and pulmonary capillary pressure ≤ 15mmHg, and perfusion defects detected by ventilation/perfusion lung scan and computed tomography pulmonary angiography). Patients were required to have received appropriate anticoagulation therapy for at least 3 months before undergoing PEA or BPA. In all patients, operability was evaluated by a multidisciplinary PH team. When surgery was contraindicated, the team considered BPA or drug therapy.8 The medication regimen in each patient group was analyzed, including use of diuretics and specific PH medication with endothelin receptor antagonists, phosphodiesterase-5 inhibitors, prostanoids, and riociguat.
The final analysis included patients with moderate-to-severe TR detected by preprocedure transthoracic echocardiography who were monitored by echocardiography after PEA or completion of the BPA program. In all patients, TR was considered a secondary functional disorder due to the absence of any structural alterations. We analyzed the last complete echocardiogram study performed in the first 12 months after the intervention; if no echocardiogram study was available for this period, we analyzed the most recent study with complete data. We excluded patients with incomplete data on the severity of postintervention TR. Hemodynamic parameters were obtained invasively by right heart catheterization. Missing values were not imputed. All study participants were included in the Spanish PH registry (Registro Español de Hipertensión Pulmonar; REHAP) and gave written informed consent in accordance with the study protocol and the ethical principles of the Declaration of Helsinki.
The patients were categorized into 2 outcome groups according to echocardiography-monitored postintervention TR status: a) absent-to-mild TR and b) moderate-to-severe TR.
We analyzed differences in clinical, echocardiographic, and hemodynamic characteristics between survivors and nonsurvivors, as well as differences in 3-year survival as a function of TR severity and the presence of significant residual PH.
Echocardiography assessmentAll patients underwent a transthoracic echocardiography examination before the intervention, and follow-up examinations were performed after a median postintervention interval of 9 [interquartile range, 5-18] months. In patients treated with BPA, this interval was calculated from the time of the final procedure. Right ventricle (RV) diastolic diameter, right atrium (RA) area, and RV function were measured by tricuspid annular plane systolic excursion (TAPSE) in 4-chamber apical view focused on the RV. Pulmonary arterial systolic pressure (PASP) was calculated as the sum of the RV-RA gradient (obtained from the peak TR velocity by applying the modified Bernoulli equation) and the mean RA pressure estimated from inferior vena cava size and dynamics. RV-arterial coupling was assessed from the TAPSE/PASP ratio.13 TR severity was determined according to the recommendations of the American Society of Echocardiography, the European Cardiovascular Imaging Association, and European valve disease guidelines applicable at the time of analysis.14,15 According to these recommendations, TR was scored as 0 (absent), 1+(mild), 2+(moderate), or 3+(severe). Moderate or severe TR was considered significant.
Statistical analysisQualitative variables are expressed as absolute frequencies and percentages. Continuous variables were tested for normal distribution with the Kolmogorov-Smirnov test; normally distributed variables are expressed as mean ± standard deviation, and those with a nonnormal distribution are expressed as the median [interquartile range]. Between-group comparisons were made by the Student t test or Mann-Whitney test for continuous variables and by the chi-square test for qualitative variables. Kaplan-Meier survival curves were compared using the log-rank test.
Univariate and multivariate logistic regression models were run to identify echocardiographic and hemodynamic predictors of persistent TR after the intervention. Postintervention mean pulmonary arterial pressure (mPAP) and pulmonary vascular resistance (PVR) were assessed using cutoff values with demonstrated prognostic value (mPAP> 30mmHg and RVP> 400dyn).16 For other variables, we first conducted a receiver operating characteristic (ROC) curve analysis to determine the best cutoff value associated with persistent moderate-to-severe TR. Variables with P values <.10 in the univariate analysis were included in the multivariate anlysis. We selected the best multivariate model that contained at least 1 hemodynamic and 1 echocardiographic variable.
Statistical significance was set at P <.05. All statistical analyses were conducted with Stata version 14.0 (StataCorp, United States).
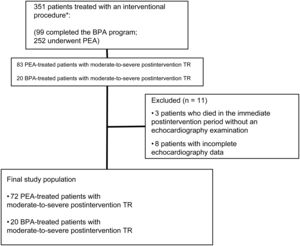
RESULTSBaseline characteristics of patients undergoing pulmonary endarterectomy or pulmonary artery balloon angioplastyDuring the study period, 252 patients underwent PEA surgery, and 99 patients completed the BPA program. The study included 92 patients who met the inclusion criteria: 72 patients who underwent PEA and 20 who had completed the BPA program (figure 1). Of the BPA patients, 4 had previously undergone PEA and had subsequently been included in an angioplasty program for patients with residual postoperative PH and poor New York Heart Association functional class. All of these patients had moderate-to-severe TR after the original PEA procedure and were assessed in the BPA treatment group. The mean age of the study population was 58.1±14.7 years, and 55% were women. Of the patients, 91.5% were in functional class III-IV before the intervention. The mean number of BPA procedures per patient was 5±2. On average, BPA patients were older (65±15.3 vs 56±13.9 years; P=.008) and had more favourable functional class. There were no significant differences between the 2 groups in preintervention exercise capacity or hemodynamic or echocardiographic characteristics (table 1 of the supplementary data).
Compared with BPA, PEA produced significantly larger reductions in mPAP and in PVR (420.0 [332.0-556.0] vs 299.0 [170.0-442.0] dyn s/cm5; P=.02). Right-chamber dimensions did not differ between the 2 groups after the intervention but the BPA group had better TAPSE-determined RV longitudinal contraction (table 1 of the supplementary data).
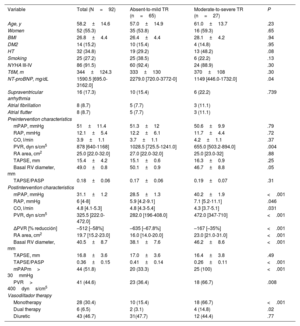
Status of postintervention moderate-to-severe tricuspid regurgitation and predictors of persistent diseaseAfter the intervention, TR persisted in 27 patients (moderate in 21, severe in 6), with no significant differences between patients treated with PEA or BPA (30.6% vs 25%; P=. 78). Absent or mild postintervention TR was associated with higher baseline PVR (1028.5 [725.5-1241.0] vs 655.0 [503.2-894.0] dyn s/cm5; P=.004) and N-terminal pro-brain natriuretic peptide (NT-proBNP). Postintervention TR status was not correlated with the remaining baseline demographic, functional, or echocardiographic characteristics. Persistent moderate-to-severe TR was associated with a worse hemodynamic profile after the intervention, characterized by elevated mPAP (40.2±1.9 vs 28.5±1.3mmHg; P <.001) and RA pressure (7.1 [5.2-11.1] vs 5.9 [4.2-9.1] mmHg; P=.04), as well as a smaller reduction in PVR from baseline than that in patients with absent-to-mild postintervention TR (–167dyns/cm5 [–35.0%] vs –635dyns/cm5 [–67.8%]; P <.001). The pattern was similar for echocardiographic variables, with persistent TR being associated with significantly smaller reductions in RA area (–8% vs –41%; P<.001) and basal RV diameter (–1% vs –24%; P <.001) (figure 1 of the supplementary data). Moderate-to-severe TR was also associated with weaker right ventricular-pulmonary artery coupling indexed by the TAPSE/PASP ratio (0.26±0.11 vs 0.41±0.14; P ≤.001). Vasodilator therapy was more intensive in patients with persistent TR than in those with absent-to-mild TR, but there was no difference in diuretic therapy. Baseline and postintervention characteristics are shown in table 1. Postintervention TR progression is shown in figure 2 of the supplementary data.
Clinical, echocardiographic, and hemodynamic characteristics of the study population stratified by the severity of postintervention tricuspid regurgitation
| Variable | Total (N=92) | Absent-to-mild TR (n=65) | Moderate-to-severe TR (n=27) | P |
|---|---|---|---|---|
| Age, y | 58.2±14.6 | 57.0±14.9 | 61.0±13.7 | .23 |
| Women | 52 (55.3) | 35 (53.8) | 16 (59.3) | .65 |
| BMI | 26.8±4.4 | 26.4±4.4 | 28.1±4.2 | .94 |
| DM2 | 14 (15.2) | 10 (15.4) | 4 (14.8) | .95 |
| HT | 32 (34.8) | 19 (29.2) | 13 (48.2) | .08 |
| Smoking | 25 (27.2) | 25 (38.5) | 6 (22.2) | .13 |
| NYHA III-IV | 86 (91.5) | 60 (92.4) | 24 (88.9) | .30 |
| T6M, m | 344±124.3 | 333±130 | 370±108 | .30 |
| NT-proBNP, mg/dL | 1590.5 [695.0-3162.0] | 2279.0 [720.0-3772-0] | 1149 [446.0-1732.0] | .04 |
| Supraventricular arrhythmia | 16 (17.3) | 10 (15.4) | 6 (22.2) | .739 |
| Atrial fibrillation | 8 (8.7) | 5 (7.7) | 3 (11.1) | |
| Atrial flutter | 8 (8.7) | 5 (7.7) | 3 (11.1) | |
| Preintervention characteristics | ||||
| mPAP, mmHg | 51±11.4 | 51.3±12 | 50.6±9.9 | .79 |
| RAP, mmHg | 12.1±5.4 | 12.2±6.1 | 11.7±4.4 | .72 |
| CO, l/min | 3.9±1.1 | 3.7±1.1 | 4.2±1.1 | .37 |
| PVR, dyn s/cm5 | 878 [640-1168] | 1028.5 [725.5-1241.0] | 655.0 [503.2-894.0] | .004 |
| RA area, cm2 | 25.0 [22.0-32.0] | 27.0 [22.0-32.0] | 25.0 [23.0-32] | .88 |
| TAPSE, mm | 15.4±4.2 | 15.1±0.6 | 16.3±0.9 | .25 |
| Basal RV diameter, mm | 49.0±0.8 | 50.1±0.9 | 46.7±8.8 | .05 |
| TAPSE/PASP | 0.18±0.06 | 0.17±0.06 | 0.19±0.07 | .31 |
| Postintervention characteristics | ||||
| mPAP, mmHg | 31.1±1.2 | 28.5±1.3 | 40.2±1.9 | <.001 |
| RAP, mmHg | 6 [4-8] | 5.9 [4.2-9.1] | 7.1 [5.2-11.1] | .046 |
| CO, l/min | 4.8 [4.1-5.3] | 4.8 [4.3-5.4] | 4.3 [3.7-5.1] | .031 |
| PVR, dyn s/cm5 | 325.5 [222.0-472.0] | 282.0 [196-408.0] | 472.0 [347-710] | <.001 |
| ΔPVR [% reducción] | –512 [–58%] | –635 [–67.8%] | –167 [–35%] | <.001 |
| RA area, cm2 | 19.7 [15.2-23.0] | 16.0 [14.0-20.0] | 23.0 [21.0-31.0] | <.001 |
| Basal RV diameter, mm | 40.5±8.7 | 38.1±7.6 | 46.2±8.6 | <.001 |
| TAPSE, mm | 16.8±3.6 | 17.0±3.6 | 16.4±3.8 | .49 |
| TAPSE/PASP | 0.36±0.15 | 0.41±0.14 | 0.26±0.11 | <.001 |
| mPAPm> 30mmHg | 44 (51.8) | 20 (33.3) | 25 (100) | <.001 |
| PVR> 400dyns/cm5 | 41 (44.6) | 23 (36.4) | 18 (66.7) | .008 |
| Vasodiltador therapy | ||||
| Monotherapy | 28 (30.4) | 10 (15.4) | 18 (66.7) | <.001 |
| Dual therapy | 6 (6.5) | 2 (3.1) | 4 (14.8) | .02 |
| Diuretic | 43 (46.7) | 31(47.7) | 12 (44.4) | .77 |
BMI, body-mass index; CO, cardiac output; DM, diabetes mellitus; HT, hypertension; mPAP, mean pulmonary arterial pressure; NYHA, New York Heart Association functional class; PASP, pulmonary artery systolic pressure; PVR, pulmonary vascular resistance; RA, right atrium; RAP, right atrial pressure; RV, right ventricle; T6M, 6-minute walk test; ΔPVR, difference between preintervention and postintervention PVR.
Data are presented as No. (%), mean ± standard deviation, or median [interquartile range].
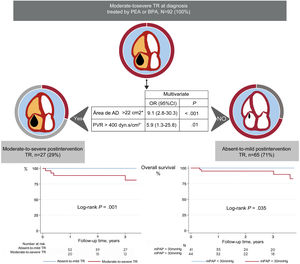
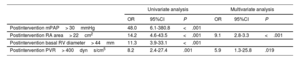
On logistic regression, none of the preintervention variables was associated with significant residual TR (table 2 of the supplementary data). Persistent TR was independently associated with postintervention PVR> 400dyns/cm5 (area below the curve=0.77; standard error=0.05; sensitivity, 64%; specificity, 74%) and a postintervention RA area> 22cm2 (area below the curve=0.83; standard error=0.05; sensitivity, 73%; specificity, 84%) (table 2).
Univariate and multivariate analysis of selected echocardiographic and hemodynamic predictors of the persistence of moderate-to-severe tricuspid regurgitation after pulmonary endarterectomy or balloon pulmonary angioplasty
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| OR | 95%CI | P | OR | 95%CI | P | |
| Postintervention mPAP> 30mmHg | 48.0 | 6.1-380.8 | <.001 | |||
| Postintervention RA area> 22cm2 | 14.2 | 4.6-43.5 | <.001 | 9.1 | 2.8-3.3 | <.001 |
| Postintervention basal RV diameter> 44mm | 11.3 | 3.9-33.1 | <.001 | |||
| Postintervention PVR> 400dyns/cm5 | 8.2 | 2.4-27.4 | .001 | 5.9 | 1.3-25.8 | .019 |
mPAP, mean pulmonary arterial pressure; PVR, pulmonary vascular resistance; RA, right atrium; RV, right ventricle
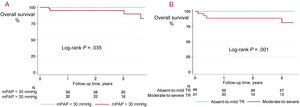
Three-year survival was 94.5% overall and was higher among patients with absent-to-mild TR after the intervention (100% vs 81.5%; P=.02). All deaths (n = 5) occurred in patients with moderate-to-severe postintervention TR: 3 patients in the PEA treatment group and 2 in the BPA treatment group. Causes of death were right heart failure in 1 patient, anticoagulation-related bleeding complications in 2 patients and causes unrelated to CTEPH in 2 patients. Compared with survivors, nonsurvivors had higher postintervention pulmonary pressure and basal RV diameter, as well as worse right ventricular-pulmonary artery coupling indexed by the TAPSE/PASP ratio. There were no significant differences between survivors and nonsurvivors in baseline characteristics (figure 2), diuretic treatment (48.3% vs 80.0%; P=.16), or pulmonary vasodilator therapy (35.4% vs 80.0%; P=.73). Three-year mortality was increased only in patients with persistent moderate-to-severe TR after the intervention (P≤.001) and in those with mPAP ≥ 30mmHg (P=.035) (figure 3).
Differences between survivors and nonsurvivors according to (A) postintervention pulmonary vascular resistance, (B) basal RV diameter; (C) mPAP, and (D) TAPSE/PASP ratio. The boxes are centered on the median value, and the box dimensions represent the interquartile range. Statistical significance was set at P<.05. PASP, pulmonary artery systolic pressure; TAPSE, tricuspid annular plane systolic excursion.
The present study describes the outcome of moderate-to-severe functional TR in patients with CTEPH treated by PEA or BPA. After the intervention, TR severity was reduced by restoration of normal pulmonary pressures and reverse remodeling of the right chambers and outcomes were similar in the 2 treatment groups.
Our results highlight the importance of treating the cause of PH and of a successful outcome of the interventional procedure. Of note, none of the preintervention variables served to identify patients with an elevated risk of persistent TR. Indeed, patients with no residual postintervention TR had higher baseline RVP; however, these patients also showed a more pronounced RVP reduction in response to the intervention. Postintervention RVP and RA area were independently related to significant postintervention TR. Final RVP is a direct reflection of the success of the intervention; in contrast, right-chamber dilation reflects both the lack of reverse remodeling due to the persistence of an elevated afterload and an increased preload due to the TR itself. These results reinforce the message that reducing pulmonary pressures should be the central goal in the treatment of patients with CTEPH (figure 4).
Central illustration. The outcome of tricuspid regurgitation (TR) after pulmonary endarterectomy (PEA) or balloon pulmonary angioplasty (BPA) depended on the persistence of elevated pulmonary vascular resistance (PVR) and right atrial (RA) area. Kaplan-Meier survival curves demonstrate the higher mortality among patients with residual PH and persistent moderate-to-severe TR.
*Postintervention variables.
Three-year survival was 94% overall and was worse among patients with mPAP ≥ 30mmHg after the intervention and in the patient group with persistent moderate-to-severe TR.
Persistent postintervention tricuspid regurgitation: progression, predictors, and therapeutic approachStudies on the progression of significant TR in CTEPH have mainly focused on patients treated by PEA and have reported postintervention reductions in TR severity in as many as 70% of patients, especially those with more favourable postintervention hemodynamic profiles and right heart reverse remodeling.10–12 In our study, satisfactory TR progression was associated with significantly larger postintervention reductions in pulmonary pressures and right-chamber dimensions, even though patients in this category had higher baseline values of NT-proBNP, RV diameter, and RVP. This finding highlights the functional nature of TR in these patients, resulting from the systolic dysfunction and the maladaptive remodeling of the right chambers in response to the chronically elevated afterload. This functional character underlies the successful reversal of these changes with therapeutic strategies to reduce pulmonary pressures (PEA or BPA), which thus reduce TR severity regardless of the baseline clinical status.16–18
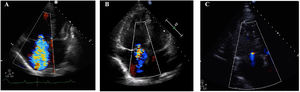
The impact of pulmonary pressure reduction on right heart reverse remodeling has been reported in the context of pulmonary artery hypertension19 and in more complex clinical situations. For example, in patients undergoing double-lung transplant, the normalization of pulmonary pressures upon transplantation resolves TR in up to 90% of organ recipients despite the presence of extensive adverse RV remodeling.20 In our cohort, the presence of PH was a prerequisite for the postintervention persistence of moderate-to-severe TR and all patients in this group had a final mPAP> 30mmHg. These observations support the view that PH plays a central role in the development of tricuspid valve disease and should therefore be the main therapeutic target. PEA and BPA have both been shown to be effective treatments for CTEPH, and these approaches can be used as complementary therapies in complex clinical situations such as residual PH after PEA.8,9,21 In our cohort, this was the situation of 4 of the patients in the BPA treatment group. The TR was resolved after normalization of the pulmonary pressures in 2 of these patients, but the hemodynamic parameters did not return to normal levels and the TR was not resolved in the remaining 2 patients. These examples illustrate the usefulness of multimodal approaches to treat persistent CTEPH and TR (figure 5).
Outcome of tricuspid regurgition (TR) in the transthoracic echocardiograms of a CTEPH patient treated first by pulmonary endarterectomy (PEA) and subsequently by balloon pulmonary angioplasty (BPA) for residual pulmonary hypertension. (A) Baseline apical 4-chamber view, showing right heart chamber dilation and severe TR; the patient's pulmonary vascular resistance was 1.188dyns/cm5. (B) Post-PEA 4-chamber view; pulmonary vascular resistance was 776dyns/cm5. (C) Post-BPA program 4-chamber view, showing reductions in right-chamber dimensions and TR severity; pulmonary vascular resistance was 298dyns/cm5.
The association between an improved hemodynamic profile and TR resolution in this study has implications for therapy. In the case of PEA, this result reaffirms the wisdom of treating TR conservatively during the index procedure, balancing the goal of resolving TR with a good hemodynamic outcome against the risk of postoperative right heart failure, which could result from the preload/afterload imbalance after TR correction.22 This situation can be exacerbated in patients with postoperative residual PH, and no associated variables have been identified to predict the occurrence of this condition,10,16 which affects up to 25% of patients treated with PEA.7 In percutaneous intervention with BPA, there are doubts about the benefits of tricuspid repair in patients with persistent TR after the intervention. Edge-to-edge repair techniques have been linked to higher 1-year mortality in patients with precapillary PH, even when the severity of their TR is reduced to a level similar to that in patients without PH. These findings show that the prognosis of patients with precapillary PH is determined by the underlying disease and not by the associated valve disease, yet again reaffirming the need to prioritize normalization of pulmonary pressures.23,24
Prognostic impact of residual tricuspid regurgitation in patients treated by pulmonary endarterectomy or pulmonary artery balloon angioplastyThe mortality in our cohort is comparable to that reported in previous studies,8,16 with worse outcomes in patients with persistent moderate-to-severe TR and PH at 3-year follow-up. The patients who died during follow-up had higher mPAP, worse right ventricular-pulmonary artery coupling, and more pronounced RV dilation, and all of these parameters were associated in our study with the persistence of TR after the intervention. These findings thus reinforce the hypothesis that TR is principally a marker of more severe underlying disease and is not an independent entity.25
LimitationsThe analysis of our results is subject to the following limitations. a) The retrospective and single-center study design could limit the external value of the results. b) The echocardiography data used to determine echocardiographic variables and grade TR severity were obtained directly from reports entered in the REHAP database when the scans were performed, without re-examination of the images. c) Although this is the largest cohort reported to date, the sample size was small, and the low event rate prevented analysis of factors associated with mortality. Despite these limitations, these results provide an up-to-date overview of TR progression in CTEPH, together with its possible therapeutic implications.
CONCLUSIONSIn patients with CTEPH and moderate-to-severe TR who underwent surgical or percutaneous treatment, the persistence of significant TR after the intervention was associated with a small reduction in afterload and with PH persistence and unfavorable right heart remodeling. Outcomes were similar in both procedures. The persistence of significant TR and the presence of residual PH were associated with a worse outcome during follow-up.
FUNDINGA. Cruz-Utrilla was supported by a Rio Hortega grant from the Instituto de Salud Carlos III (Spanish Ministry of Science and Innovation; CM20/00164).
AUTHORS’ CONTRIBUTIONSData collection: L. Gómez-Burgueño, M. Otero, W. Hinojosa. Statistical analysis: A. Cruz-Utrilla, W. Hinojosa. Manuscript writing and revision: W. Hinojosa, A. Cruz-Utrilla, T. Segura de la Cal, C. Jiménez López-Guarch, M. Velázquez-Martín, M. J. López-Gude, R. Morales, J. M. Cortina Romero, J. Solís, F. Arribas Ynsurriaga, P. Escribano-Subías.
CONFLICTS OF INTERESTNone.
- –
Little information is available on the status of moderate-to-severe TR after PEA or BPA. Unresolved questions are which factors are associated with persistent TR after intervention and whether patients with this condition could be candidates for surgical or percutaneous tricuspid valve repair.
- –
Outcomes in moderate-to-severe TR in patients with CTEPH depend on postintervention hemodynamics and right heart reverse remodelling and are similar in patients treated with PEA or BPA.
- –
Preintervention variables do not identify patients with a high risk of persistent postintervention TR.
- –
Postintervention moderate-to-severe TR and residual PH are associated with poor outcomes during follow-up.
Supplementary data associated with this article can be found in the online version, at https://doi.org/10.1016/j.rec.2023.02.016