Most of the signs and symptoms of heart failure can be explained by fluid overload, which is also related to disease progression. Fluid overload is a complex phenomenon that extends beyond increased intravascular pressures and poses challenges for accurate diagnosis and effective treatment. Current recommendations advise a multiparametric approach, including clinical data (symptoms/signs), imaging tests, and biomarkers. This article proposes a practical therapeutic approach to managing hydrosaline overload in heart failure in both inpatient and outpatient settings. This document is an initiative of the Spanish Society of Internal Medicine (SEMI) in collaboration with the Spanish Society of Cardiology (SEC) and the Spanish Society of Nephrology (S.E.N.).
Keywords
This document is an initiative of the Spanish Society of Internal Medicine in collaboration with the Spanish Society of Cardiology and the Spanish Society of Nephrology. A group was formed comprising 18 experts in the management of patients with heart failure (HF) (6 internists, 6 cardiologists, and 6 nephrologists). The group was coordinated by a clinician from each specialty. Topics of interest were selected and distributed among 3 working groups, each comprising 6 members, 2 from each specialty. The recommendation levels of the document mirror those of the clinical practice guidelines.1 The group of experts assessed, discussed, and validated aspects lacking relevant recommendations in the clinical practice guidelines.
INTRODUCTIONVolume expansion is a central component in the definition of worsening HF.1 Although diuretics lead to clinical improvement in most patients with HF, the response varies. Accordingly, it is essential to explore in greater depth the pathophysiology of the fluid overload to optimize and individualize its management.2,3
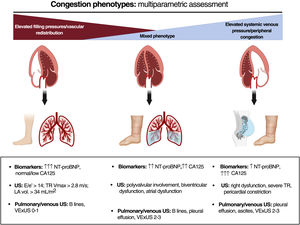
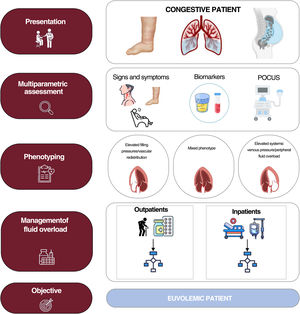
The current evidence suggests that fluid overload exhibits considerable variability in terms of severity, quantity, and distribution.3 This is due to the complex pathophysiology of the condition, with multiple interconnecting mechanisms: cardiac anatomy and function and changes in pulmonary and systemic venous capacitance, vascular endothelial barrier function, neurohormonal activation, interaction between hydrostatic and oncotic pressures in the capillary and interstitial compartments, and lymphatic system integrity and distensibility.3 All of the above can result in a disconnection between the pressure and fluid overloads and give rise to distinct clinical and hemodynamic patterns.4 Thus, volume redistribution from the splanchnic to pulmonary vascular territory predominates in some patients while endovascular/interstitial fluid overload predominates in others. An updated view of the congestion/volume expansion necessitates a more exhaustive classification based on onset (acute vs chronic), regional distribution (systemic vs pulmonary), distribution compartment (endovascular vs interstitial), and a distinction between clinical and subclinical.5 A accurate identification of these phenotypes via a multiparametric approach will undoubtedly improve the management of HF3 (figure 1). In addition, this focus will permit the detection of residual or subclinical congestion that can be present at hospital discharge or after the initial treatment during an outpatient decompensation and that is associated with worse patient prognosis, both in terms of mortality and HF readmissions. Decongestion detection and management have been improved by the multiparametric approach, which combines signs and symptoms mainly obtained with point-of-care ultrasound and blood tests (natriuretic peptides, carbohydrate antigen 125, hematocrit). Moreover, carbohydrate antigen 125-guided treatment in both hospitalized patients and outpatients has been shown to improve patient prognosis. Lung ultrasound could also help to guide the management of patients with acute heart failure (AHF), as has been found in randomized clinical trials.6,7 Accordingly, we believe that the ultrasound and blood test parameters for decongestion could be useful tools for minimizing the residual congestion and guiding the diuretic therapy in our patients3,5 (figure 2).
The objective of this document is to summarize the recommendations, based on scientific evidence and the opinions of a panel of experts, on the management of fluid overload in both inpatient and outpatient settings by proposing a practical therapeutic approach developed by a panel of experts from the Spanish Society of Internal Medicine, the Spanish Society of Cardiology, and the Spanish Society of Nephrology.
MANAGEMENT OF FLUID OVERLOADLoop diureticsLoop diuretics are the main treatment line for volume overload in HF.2 The most commonly used member of this family is furosemide, although its bioavailability is highly variable and erratic when orally administered.2 Accordingly, drugs with a better theoretical profile, such as bumetanide and torsemide, could have advantages. However, the TRANSFORM-HF study failed to show differences in long-term clinical benefits between furosemide and torsemide.8
The best evidence currently available on the dosage of loop diuretics in AHF has been provided by the DOSE-HF trial.9 That study compared 2 different strategies: first, intravenous furosemide in twice-daily boluses vs continuous infusion; and second, low-dose furosemide (equivalent to the patient's previous oral dose) vs high-dose furosemide (2.5 times the previous oral dose). For the comparison between the bolus and the continuous infusion, no significant differences were found in the global assessment of symptoms or in the mean change in creatinine levels. The high-dose strategy was associated with a nonsignificant tendency for symptom improvement and greater diuresis, weight loss, and net fluid loss at 72hours but also with a transient worsening of renal function.9
Route of administrationIn patients with AHF, the current recommendation is to use a stepped and progressive intravenous diuretic therapy based on the diuretic response, starting with high intravenous doses (twice the daily baseline oral dose) and progressively decreasing the dose until the minimal effective oral dose is reached.2
In addition to the oral and intravenous routes, subcutaneous administration is an option. A small clinical trial performed in patients with AHF treated on an outpatient basis found no significant differences in diuresis at 6hours between the intravenous and subcutaneous routes, using a new subcutaneous formulation of furosemide with a neutral pH.10
Observational studies have also revealed a benefit of conventional furosemide.11,12
Thiazide diureticsThiazides inhibit the sodium-chloride cotransporter in the distal convoluted tubule2 and less potently remove fluid than loop diuretics, by acting on distal segments of the nephron. The compensatory increase in sodium and water reabsorption in the distal segments with loop diuretics is why thiazides could be beneficial in loop diuretic-resistant patients. This hypothesis is supported by the results of the recent CLOROTIC trial.13 In that study, 230 patients hospitalized for AHF were randomized to switch from 80mg or more of oral furosemide to hydrochlorothiazide HCTZ or placebo, in addition to a protocol-based intravenous furosemide regimen. The patients who received HCTZ exhibited greater weight loss and decongestion at 72hours but worse hyperkalemia and renal function deterioration. However, as in the DOSE-HF trial,9 this decline did not result in a worse prognosis,11 although the study was not designed or powered to analyze this effect. Recently, a substudy of the CLOROTIC trial showed that combination diuretic and thiazide therapy is safe and effective for the entire spectrum of renal function.14
AcetazolamideAcetazolamide (ACZ) is a diuretic that acts on the proximal convoluted tubule and blocks the action of carbonic anhydrase, which converts carbon dioxide and water into carbonic acid.2 ACZ therapy has been evaluated in patients with acute congestive HF who are refractory to diuretic therapy.2 The most robust and recent evidence is derived from the ADVOR clinical trial, which randomized 519 patients with AHF and evidence of fluid overload.15 In that study, the addition of intravenous ACZ to loop diuretic therapy improved decongestion, natriuresis, and diuresis in the short term but failed to decrease hospitalizations and mortality in the long term.15 In this way, intravenous ACV (500mg/d) could be considered in patients with AHF in conjunction with intensive treatment with loop diuretics.
Mineralocorticoid receptor antagonistsThe effect of mineralocorticoid receptor antagonists (MRAs) on the diuretic response in AHF is controversial.2,16 The main trial of MRAs in AHF, the ATHENA-HF study,16 showed neutral results. In that study, 360 patients were randomized to receive spironolactone (100mg/d) vs placebo or low-dose spironolactone (12.5 or 25mg/d) for 96hours, together with a loop diuretic. Although no adverse events were recorded, there were no differences in N-terminal pro-B-type natriuretic peptide (NT-proBNP) levels, diuretic efficacy parameters, mortality, or HF decompensation. A possible explanation is the slow conversion of spironolactone to its active metabolites and the treatment of 25% of the patients of the placebo group with 25mg spironolactone.16 In a nonrandomized study comparing natriuretic and diuretic efficacy in patients with AHF and left ventricular ejection fraction ≥ 50% receiving intravenous loop diuretics, the administration of chlorthalidone vs spironolactone was associated with greater short-term natriuresis and diuresis.17
MRAs can be considered for this purpose, as well as for treating patients with left ventricular dysfunction (left ventricular ejection fraction < 40%) and patients with diuretic resistance despite receiving combined treatment with optimal doses of loop diuretics, thiazides, or proximal tubule diuretics, largely in the presence of hypokalemia.
AquareticsTolvaptan is a selective vasopressin receptor 2 antagonist. The use of this drug has been evaluated in AHF, at an oral dose of 30mg, in 3 clinical trials: EVEREST,18 TACTICS-HF,19 and SECRET-HF.20 Regarding standard diuretic therapy, tolvaptan managed in all of the studies to reduce weight and achieve diuresis significantly better than placebo in the short term. However, its use did not result in improvements in mortality or readmission in the mid-to-long term. A substudy of the EVEREST trial suggested a significant increase in plasma sodium levels, improved decongestion, and a greater reduction in adverse clinical events in patients with hyponatremia.21 Tolvaptan is safe and causes no major hemodynamic changes and can be safely and effectively administered to patients with advanced kidney disease.22 In patients with AHF under treatment with an intensive diuretic regimen, the addition of tolvaptan could be a therapeutic option in the presence of hyponatremia.
Finally, oral urea (15-30g) is a drug with proven aquaretic properties.23 Although it could be considered an aquaretic option, intervention studies are specifically required in patients with HF.
Sodium-glucose cotransporter-2 inhibitorsBeyond the cardiorenal benefits of sodium-glucose cotransporter 2 inhibitors (SGLT2is),24–29 their role as diuretic agents is less impactful. SGLT2is exert a moderate natriuretic effect and exhibit predominant aquaretic properties.30 Their efficacy in AHF is supported by various clinical trials whose primary objectives were to analyze the effect of SGLT2is on prognosis, with a minor interest in their diuretic effects. The SOLOIST-WHF trial, which included only patients with diabetes, is thus far the largest study to examine the safety and efficacy of sotagliflozin vs placebo.31 The results showed that sotagliflozin, started before or soon after hospital discharge, exhibited a good safety profile and was associated with significantly fewer cardiovascular deaths, hospitalizations, and emergency room visits for HF vs placebo.31 The EMPULSE study compared empagliflozin 10mg/d with placebo in patients with initially stable AHF.32 The results showed that empagliflozin initiation is well tolerated in this context, with a significant net clinical benefit at 90 days after treatment initiation.32
Thus far, the most important study to analyze the diuretic effect of SGLT2is in the acute setting is the DAPA-RESIST trial,33 which compared dapagliflozin 10mg/d vs metolazone 5-10mg once daily for a 3-day treatment period in patients hospitalized for AHF and resistant to intravenous furosemide treatment, randomized before 24hours of their hospital stay. The primary endpoint (change in weight) was not different at 5 days, although a higher total dose of furosemide was required in the dapagliflozin group and diuretic efficacy was lower than in the metolazone group. In addition, dapagliflozin exhibited a good safety profile and fewer biochemical disturbances (renal function, sodium, and potassium).
Based on the available evidence, all patients with chronic and acute HF are recommended to be treated with SGLT2is, unless contraindicated. In the acute phase, the evidence indicates that these drugs can be started in the first hours of exacerbation onset, not due to their diuretic power but for their prognostic benefits.
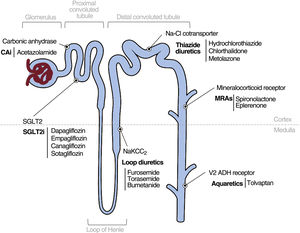
The above-mentioned drugs and their properties are summarized in table 1 of the supplementary data and are presented in figure 3.
Other therapiesFurosemide plus hypertonic saline solutionRefractoriness to diuretic therapy is associated with a higher risk of mortality and acute kidney injury.2,3 This condition is common in patients with the predominant peripheral congestion phenotype, who would theoretically benefit from increased transport of sodium and water to the endovascular space.
A double-blind clinical trial34 including 94 patients with refractory HF with left ventricular systolic dysfunction compared high doses of intravenous furosemide (500-1000mg/d) vs the same dose of furosemide plus hypertonic saline solution (HSS) infusion. The results showed greater weight loss, a larger reduction in natriuretic peptides, a shorter hospital stay, and a lower risk of 30-day readmission in the group treated with HSS.34 The SMAC-HF clinical trial,35 which included 1927 patients with AHF, reduced left ventricular ejection fraction, and New York Heart Association functional class III, revealed that patients who received HSS during hospitalization had better diuresis, a shorter length of hospital stay, and reductions in readmission and mortality at 57 months vs patients who received placebo, proving the clinical safety of HSS.35 The limitations of this treatment are the need to use very high doses of furosemide and the heterogeneity of the sodium chloride concentration used, which ranges between 1.4% and 7.5%. This suggests that this strategy should be reserved for patients with refractory congestion who are receiving intensive diuretic therapy.
Extracorporeal ultrafiltration techniques and continuous ambulatory peritoneal dialysisThe most recent European clinical practice guidelines recommend the use of renal replacement therapy in patients with diuretic resistance and persistent fluid overload or with marked deterioration in renal function (class IIa recommendation).1
Ultrafiltration (UF) involves the removal of plasma water via a semipermeable membrane with a transmembrane pressure gradient.36 The treatment can be performed using conventional hemofiltration, hemodialysis, and peritoneal dialysis techniques or with UF devices with peripheral venous access. The most noteworthy potential advantages of UF compared with diuretics include the greater removal of sodium because the ultrafiltrate is isotonic with plasma (134-138 mmol/L of ultrafiltrate), in contrast to hypotonic fluid removal with diuretics, and a more controlled liquid removal rate.36 A recent meta-analysis including the main clinical trials comparing both strategies concluded that UF is safe but does not reduce the risk of mortality or readmission.37
Another UF option for the more long-term management of congestive HF refractory to diuretic therapy is continuous ambulatory peritoneal dialysis. In refractory HF, this form of dialysis has exhibited long-term clinical, functional, and prognostic benefits.38 However, no randomized studies of this strategy have been performed.
Inotropic agents and vasopressorsInotropic agents and vasopressors are reserved for situations in which the fluid overload is accompanied by peripheral hypoperfusion. According to the recommendations in the clinical practice guidelines, inotropic agents can be considered to improve peripheral perfusion and maintain organ function in patients with systolic blood pressure < 90mmHg and evidence of hypoperfusion who do not respond to standard treatment (class IIb, level of evidence C).1 Vasopressors can be an option to increase blood pressure and maintain vital organ perfusion in patients with cardiogenic shock (class IIb, level of evidence B).1
TREATMENT ALGORITHM FOR FLUID OVERLOAD IN ACUTE HEART FAILUREHospitalized patientsThe treatment of fluid overload in patients hospitalized for AHF requires the appropriate diagnostic approach and correct etiology (fluid overload or vascular redistribution), an accurate assessment of the systemic renal and hemodynamic situation, and an understanding of the different therapeutic options.
Patients with predominant fluid overload should be administered intensive diuretic therapy; if vascular redistribution predominates, the diuretic therapy will have to be less intensive and be combined with vasodilators2 (table 2 of the supplementary data). In clinical situations complicated by peripheral hypoperfusion, the use must be considered of inotropic agents or vasopressors1 (table 2 of the supplementary data).
First, the severity of the patients’ fluid overload must be established (table 1), as well as whether baseline diuretic therapy is being received and, if so, at what dose. The scale awards a score of 0 to 3 for each of the 10 variables, giving a total score of 30. For the fluid overload to be considered moderate or severe, the presence is required of at least 1 maximum value in each category (clinical, biomarkers, and ultrasound). The treatment objective must be euvolemia, based on the scale for assessing fluid overload (table 1), with a score ≤ 4 recommended.
Fluid overload grades based on a multiparametric approach
| Variables | Euvolemia | Mild | Moderate | Severe |
|---|---|---|---|---|
| Clinical variables | ||||
| Orthopnea | No | 1 pillow | 2 pillows | Persistent |
| JVD, cm | < 6 | 6-9 | 9-15 | > 15 |
| Crackles | Absent | Bases | < 50% | > 50% |
| Edema | Absent | Ankles | Knees | > Knees |
| Ascites | No | Minimal, not requiring paracentesis | Moderate, amenable to paracentesis | Tense, requiring paracentesis |
| Biomarkers | ||||
| CA125, U/mL | < 20 | 20-34 | 35-99 | > 100 |
| NT-proBNP, pg/mL | < 100/< 300 | 100-400/300-1800 | 400-2500/1800-10 000 | > 2500/> 10 000 |
| Lung ultrasound | ||||
| Pleural effusion | Absent | < 1 cm | > 1 cm | Atelectasis |
| B-lines | Absent | < 3 lines per field | > 3 lines in less than 2 regions per affected lung | > 3 lines in 2 regions per affected lung |
| VExUS | 0 | 1 | 2 | 3 |
BNP, B-type natriuretic peptide; CA125, carbohydrate antigen 125; JVD, jugular venous distention; NT-proBNP, N-terminal pro-B-type natriuretic peptide; VExUS, venous excess ultrasound.
The main diuretic for the initial treatment of fluid overload is intravenous furosemide1,2,39 (class I, level of evidence B). In patients not previously treated with diuretics, the ideal starting dose is 20 to 40mg of intravenous furosemide in a single bolus.1,2,39 Otherwise, the patients’ dose must be double their usual dose.1,2,39 It must be remembered that patients with chronic kidney disease, particularly those with more advanced stages (estimated glomerular filtration rate < 30mL/min/1.73 m2), will show lower tubular diuretic concentrations and, consequently, less of a response. Accordingly, the initial dose must be 2 to 3 times higher than that in patients with normal renal function.2
Patients should urinate and empty their bladder before being administered the furosemide bolus. The furosemide should be administered immediately afterward and a urine sample should be obtained at 2hours for the analysis of urine sodium levels.1 Sodium values < 70 mmol/L suggest an insufficient diuretic response to the first dose administered.1,2,40 We must also assess the diuresis at 6hours, considering values < 100 to 150mL/h to indicate a weak diuretic response. If the urine sodium at 2hours is < 70 mmol/L or the diuresis at 6hours is < 100mL/h, the furosemide bolus should be doubled, with a 12-hour administration schedule (another consideration is continuous infusion with a total daily dose not exceeding 400-600mg/d); these measures are priorities in patients with renal dysfunction.2 The PUSH-AHF clinical trial showed that urine sodium-guided diuretic therapy vs the standard approach resulted in better natriuresis and diuresis, with no differences in clinical events (total mortality or HF hospitalizations) at 6 months.41
Sequential nephron blockadeA second diuretic is recommended at early stages, mainly if the fluid overload is highly patent or the patient has already been treated with baseline doses of diuretics and has not responded adequately to the initial bolus.1 In this setting, we can use distal diuretics such as HCTZ and chlorthalidone (there is no evidence from trials for chlorthalidone) or proximal diuretics such as intravenous ACZ (if intravenous administration is not possible, oral administration is a possibility, although the evidence is weaker). Thiazide should be prioritized in patients previously treated with outpatient furosemide therapy at ≥ 80mg/d 13 who do not have hyponatremia. ACZ should be prioritized in patients receiving lower baseline doses of furosemide as outpatients and with elevated levels of bicarbonate and hypochloremia.15,41 Oral potassium supplementation is recommended in patients treated with thiazides, mainly those with plasma potassium levels < 4 mmol/L; caution must be exercised in individuals with renal function deterioration.
The diuretic response must be reassessed at 24hours.2 The assessment should include clinical progression, diuresis, ultrasound, and biomarkers. If there is no clinical improvement and the diuresis is < 100mL/h, the intravenous furosemide must be doubled and the second diuretic unchanged.13,15 If the clinical course and diuresis rhythm are adequate (> 100mL/h), the dosage schedule should be maintained. At 48hours, a multiparametric assessment of the congestion is required. If the congestion improves or has resolved, the diuretic therapy can be stepped down. However, if the response is not satisfactory, a third diuretic should be added (thiazide or ACZ, depending on the initial choice).
At 72hours, a new assessment should be performed and the previous procedures repeated.
If the fluid overload persists, a fourth diuretic (an aquaretic) can be considered or intensive diuretic therapy plus HSS infusion. Both strategies are priorities in patients with hyponatremia, and thiazides are to be avoided in this situation.
Importantly, hospitalized patients receiving intravenous diuretic therapy must undergo monitoring of their vital signs and fluid balance every 8hours, in addition to a daily weight assessment, although strict salt and water restriction is not required.
If, after all of these measures have been taken and other correctable causes of the refractoriness have been ruled out, such as low output or dietary lapses during admission, patients do not yet show a resolution of the fluid overload or life-threatening condition, the use of UF techniques must be considered.1,37,42 If the patient is not indicated for these aggressive treatments, palliative measures to ameliorate symptoms should be applied.
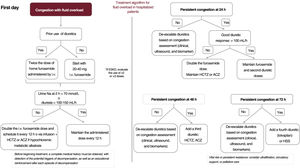
This inpatient algorithm is illustrated in figure 4.
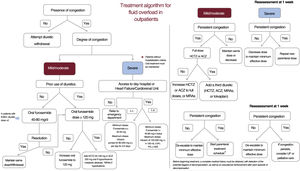
OutpatientsLess evidence is available on the management of fluid overload in the outpatient setting. First, a multiparametric approach must be used to establish the severity and regional and compartmental distribution of the fluid overload in each patient (figure 1). In patients not showing signs of volume overload or congestion, diuretic therapy is recommended, starting from a low dose and with a gradual increase; withdrawal should only be considered in euvolemic patients after 6 months without decompensation and with stable oral diuretic therapy (40-80mg furosemide) (class I, level of evidence B).1,43 Patient education and empowerment are critical in the outpatient diuretic therapy algorithm.
In line with the schematic for hospitalized patients, we must also determine in outpatients if the fluid overload is mild, moderate, or severe. To do so, we use congestion scores that include clinical variables, biomarkers, and imaging tests (table 1).
Loop diuretics are the medication of choice,2 although the dose depends on any previous exposure to these drugs and the patient's clinical status.
In patients with mild or moderate congestion with previous diuretic therapy, treatment is started with oral furosemide at 40 to 80mg/d with reassessment after 1 week. If the patient is already taking diuretics, the oral furosemide dose is increased to 120mg/d in 2 doses. In patients already taking ≥ 120mg of oral furosemide per day, the nephron must be blockaded at other levels through the addition of a second diuretic (HCTZ, ACZ, or MRA). The HCTZ dose must be adjusted to renal function (estimated glomerular filtration rate > 50mL/min/1.73 m2, 25mg/d; 20-50mL/min/1.73 m2, 50mg/d; and < 20mL/min/1.73 m2, 100mg/d).13,14 The oral starting dose of ACZ is 250mg/d while the maximum is 500mg/d.44 The use of thiazides is prioritized as second diuretic, except in patients with hypochloremic metabolic alkalosis, for whom ACZ is preferred.45 If hyperkalemia is present, MRAs can be considered as potassium-sparing diuretics.
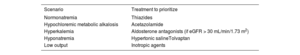
If early effective decongestion is achieved, the second diuretic is withdrawn and the baseline dose of furosemide is continued. If the response is satisfactory but slower and the patient has a heart disease that increases the risk of congestion (eg, severe tricuspid regurgitation), the thiazide or ACZ can be maintained in the long term at lower doses. In contrast, if the fluid overload persists, the dose of the second diuretic is increased; if it is already at the maximum dose, we add a third diuretic (thiazides, ACZ, or MRAs). Patients with hyponatremia and predominantly peripheral fluid overload should be treated as a priority with SGLT2i. When, in addition to peripheral fluid overload predominance, there is hyponatremia, the drugs to consider are aquaretics (tolvaptan 15-30mg/d or urea 15-30g/d). The scenarios in which each treatment should be prioritized are shown in table 2.
Scenarios in which a specific treatment is prioritized
| Scenario | Treatment to prioritize |
|---|---|
| Normonatremia | Thiazides |
| Hypochloremic metabolic alkalosis | Acetazolamide |
| Hyperkalemia | Aldosterone antagonists (if eGFR > 30 mL/min/1.73 m2) |
| Hyponatremia | Hypertonic salineTolvaptan |
| Low output | Inotropic agents |
eGFR, estimated glomerular filtration rate.
All patients under treatment with an intensive diuretic regimen (multinephron blockade or parenteral treatment) require a strict clinical and biochemical follow-up. Modification of the diuretic therapy in these patients demands strict monitoring in the subsequent week, with a focus on renal function and electrolytes.1 In patients with persistent congestion despite the above-described measures, a parenteral treatment regimen is to be started in a specialized unit, with systems permitting its outpatient administration. If these units are not available and the primary care team has no experience with the management of these patients, they will need to be referred to an emergency department to receive the parenteral treatment.
Patients with severe congestion must receive parenteral treatment, whether subcutaneous or intravenous, with the latter a priority if clinically and logistically permitted. The subcutaneous furosemide dose ranges from 20 to 40mg/d to infusion pumps that deliver 80 to 250mg/d for 3 to 5 days in more severe patients or those with difficulty accessing HF units. The intravenous furosemide dose can range from 80mg boluses to infusions of 125 or 250mg/d (with or without HSS) in the most severe patients.
Patients require an early clinical, blood test, and ultrasound reassessment in the first week. The actions required in this early evaluation are summarized in table 3. The clinical congestion scale awards a score of 0 to 3 for each of the 5 variables, giving a maximum score of 15. Patients should have a score ≤ 2 during follow-up (table 4).
Actions to perform in the early reassessment
| Variable | At 7-14 d |
|---|---|
| Clinical congestion scale | |
| POCUS | |
| VExUS | |
| LUS | |
| RGF, Na/Cl/K | |
| Complete blood count | |
| CA125 | |
| NPs | |
| Ions in urine | |
| uACR |
CA125, carbohydrate antigen 125; Cl, chloride; K, potassium; LUS, lung ultrasound; Na, sodium; NPs, natriuretic peptides; POCUS, point-of-care ultrasound; RGF, renal glomerular filtration; uACR, urine albumin:creatinine ratio; VExUS, venous excess ultrasound.
Clinical congestion scale
| Variable | Euvolemia | Mild | Moderate | Severe |
|---|---|---|---|---|
| Orthopnea | No | 1 pillow | 2 pillows | Persistent |
| Jugular venous distention, cm | < 6 | 6-9 | 9-15 | > 15 |
| Crackles | Absent | Bases | < 50% | > 50% |
| Edemas | Absent | Ankles | Knees | > Knees |
| Ascites | No | Minimal, not requiring paracentesis | Moderate, amenable to paracentesis | Tense, requiring paracentesis |
In general, this type of parenteral strategy should preferably be performed in an outpatient setting (external clinic, day hospital, or HF or cardiorenal unit), as long as the patient shows no criteria for hospitalization, such as dyspnea at rest, acute respiratory failure, hemodynamic instability, and a nonresponse to outpatient parenteral regimens.
Patients can eventually become resistant or refractory to the measures applied. Before we consider this to be the case, we must rule out other causes, such as poor adherence to hygienic and dietary measures, a lack of treatment adherence, renal hypoperfusion due to excessive use of vasodilators, and the contribution of low cardiac output in patients with left ventricular dysfunction, who would benefit from, for example, inotropic agents.
The available options to be considered in patients resistant to outpatient regimens include the use of UF techniques, such as hemodialysis or peritoneal dialysis, as well as circulatory support. Mechanical circulatory support or heart transplant should be considered in selected patients with advanced HF refractory to optimal medical therapy. If the patient is not indicated for these treatments, palliative measures to ameliorate symptoms should be applied. This outpatient algorithm is shown in figure 5.
Treatment algorithm for fluid overload in outpatients. ACZ, acetazolamide; ESKD, end-stage kidney disease; HCTZ, hydrochlorothiazide; HSS, hypertonic saline solution; i.v., intravenous; MRAs, mineralocorticoid receptor antagonists; PS, physiological saline; s.c., subcutaneous; UF, ultrafiltration.
The present consensus document has some limitations. First, primary care professionals did not contribute to its drafting. Second, implementation of the recommendations could be limited in medical care settings that have not adopted the multiparametric assessment of fluid overload. Third, the management of HF is constantly evolving and requires continuous updating. Finally, further studies are required to reveal if diuretic strategies depend on the patient's sex.
FUNDINGThis work was partly funded by grants from the Centro de Investigación Biomédica en Red en Enfermedades Cardiovasculares (CIBERCV) (grant number, 16/11/00420).
STATEMENT ON THE USE OF ARTIFICIAL INTELLIGENCEArtificial intelligence has not been used in the preparation of this article.
AUTHORS’ CONTRIBUTIONSP. Llàcer, G. Romero, and J. Núñez: article drafting and final approval. J. C. Trullàs, R. de la Espriella, M. Cobo, B. Quiroga, J. Casado, M. F. Slon-Roblero, J. L. Morales-Rull, J. I. Morgado, A. Ortiz, F. Formiga, M. Melendo-Viu, P. de Sequera, A. Recio, J. Díez, and L. Manzano: critical revision of the intellectual content and final approval.
CONFLICTS OF INTERESTNone.
Supplementary data associated with this article can be found in the online version, at https://doi.org/10.1016/j.rec.2024.01.008.