The aim of this study was to evaluate the effect of aliskiren on aortic stiffness in patients with Marfan syndrome (MS).
MethodsTwenty-eight MS patients (mean age ± standard deviation: 32.6 ± 10.6 years) were recruited from November 2009 to October 2014. All patients were receiving atenolol as standard beta-blocker therapy. A prospective randomization process was performed to assign participants to either aliskiren treatment (150-300mg orally per day) or no aliskiren treatment (negative control) in an open-label design. Central aortic distensibility and central pulsed wave velocity (PWV) by magnetic resonance imaging (MRI), peripheral PWV, central aortic blood pressure and augmentation index by peripheral tonometry, and aortic dilatation by echocardiography were examined initially and after 24 weeks. The primary endpoint was central aortic distensibility by MRI.
ResultsIn analyses of differences between baseline and 24 weeks for the aliskiren treatment group vs the negative control group, central distensibility (overall; P = .26) and central PWV (0.2 ± 0.9 vs 0.03 ± 0.7 [m/s]; P = .79) by MRI were not significantly different. Central systolic aortic blood pressure tended to be lower by 14mmHg in patients in the aliskiren treatment group than in the control group (P = .09). A significant decrease in peripheral PWV (brachial-ankle PWV) in the aliskiren treatment group (–1.6 m/s) compared with the control group (+0.28 m/s) was noted (P = .005).
ConclusionsAmong patients with MS, the addition of aliskiren to beta-blocker treatment did not significantly improve central aortic stiffness during a 24-week period.
Keywords
Marfan syndrome (MS) is an inherited connective tissue disorder caused by a mutation of the fibrillin-1 gene (FBN1), resulting in morbidity and mortality due to aortic dilatation and dissection.1,2 Therapy with beta-adrenergic blockers has been proposed as the standard treatment for MS. However, the degree of aortic dilatation and response to beta-blockers vary among adults with MS.3
Abnormal or deficient fibrillin-1 probably affects the structural integrity of the extracellular matrix and may enhance the release of active transforming growth factor-beta (TGF-β),4 resulting in aberrant thickening of the aortic media with fragmentation and disarray of elastic fibers. Transforming growth factor-beta mediates disease pathogenesis in MS and leads to aortic stiffness. Cross-talk between the TGF-β system and the renin-angiotensin system (RAS) has been demonstrated. The angiotensin receptor blocker (ARB) losartan is known to inhibit TGF-β signaling, and the development of pathologic changes in the aortic wall and progressive dilation of the aortic root were attenuated or prevented by systemic treatment with a TGF-β neutralizing antibody or ARB in an MS mouse model.5,6 In a small cohort study, the use of ARB therapy (losartan or irbesartan) significantly slowed the rate of progressive aortic dilatation in patients with MS after beta-blocker therapy had failed to prevent aortic root dilatation.7 However, large randomized trials showed discrepancies in the effect of losartan on aortic growth and the real effect of losartan is still debated in MS patients.8–11
Aliskiren is a direct renin inhibitor and a new class of renin-angiotensin-system blockers. There is some evidence that aliskiren suppresses the expression and production of TGF-β in in vitro, in vivo and clinical studies.12–14 To date, no prospective randomized clinical trial has investigated the benefit of aliskiren in the treatment of MS.
Arterial stiffness is a known predictor of aortic dilatation15 and cardiovascular complications in various cardiovascular diseases.16 Arterial stiffness is one of the earliest detectable signs of functional and structural changes in the vascular wall.17–19 Antihypertensive treatments have shown improved aortic stiffness in short-term trials lasting less than 4 weeks.20–22 Aortic distensibility, central pulse wave velocity (PWV), carotid-femoral PWV, and the augmentation index are useful parameters for assessing aortic stiffness.23,24 Aortic stiffness is related to aortic growth and prognosis in MS. Beta-blocker therapy in MS reduced aortic distensibility and PWV.25 Both aortic diameter and aortic distensibility are independent predictors of progressive aortic dilatation in MS.15 Regional central PWV and aortic distensibility are increased in MS patients and have shown moderate to high specificity for predicting the absence of regional aortic luminal growth, with proven prognostic value.26 Therefore, aortic stiffness is a logical therapeutic target in adults with MS.
In this study, we investigated whether aliskiren significantly decreases aortic stiffness compared with negative control in patients with MS under atenolol treatment.
METHODSStudy Design and Study PopulationThis was a prospective randomized study conducted in a single center. A randomization process was performed to assign participants to either the aliskiren treatment group or the negative control group in an open-label design. The duration of the study period was 24 weeks. Aliskiren was administered to patients in the treatment group at an oral dose of 150 to 300mg per day. Medication administration started after a baseline study with a dose of 150mg of aliskiren, which was escalated to 300mg of aliskiren at 4 weeks after evaluation of tolerability and the presence of adverse events. The patients stopped taking aliskiren if serious adverse events developed. Dose reduction was considered in patients who developed hyperkalemia, elevation of serum creatinine to twice baseline levels, symptomatic hypotension, gout, or renal stones. Dose reduction to 150mg after escalation was performed on the decision of the investigators if the patient complained of discomfort and adverse effects that were probably related to the medication.
Marfan syndrome patients were recruited at Samsung Medical Center from November 2009 to October 2014. All patients were receiving atenolol as standard beta-blocker therapy. All patients gave written informed consent to participate in the study, which was approved by the Institutional Ethics Committee. This trial was registered at ClinicalTrial.gov (Identifier: NCT01715207).
Inclusion criteria were age 14 to 55 years, a diagnosis of MS by Ghent criteria, beta-blocker treatment for at least 3 months, and no chronic RAS inhibitor therapy (ie, ARBs or angiotensin-converting enzyme inhibitors) for 90 days prior to screening. Exclusion criteria were a previous medical history of aortic surgery and/or dissection, significant valve disease requiring surgery, aortic root dimension > 5.0cm, renal dysfunction (creatinine > upper normal limit), pregnancy or planned pregnancy within 12 months of study entry or current breast feeding, known renal artery stenosis, hypersensitivity to aliskiren or any of the excipients, elevation of serum creatinine during follow-up (> 30% of baseline), diarrhea resulting in severe dehydration, development of gout or ureter stone, symptomatic hypotension (systolic blood pressure < 90mmHg with symptoms), hyperkalemia, and concomitant treatment with cyclosporin A.
Follow-up and OutcomesAll included patients were clinically followed up to monitor adverse effects of angioedema, gastrointestinal symptoms, rash, gout, hypotension, and renal stones at the initial examination, 1 week, 4 weeks, 8 weeks, 16 weeks, and 24 weeks. The following laboratory data were collected during the same period: potassium, electrocardiogram, creatinine, uric acid, and urine analysis. Echocardiographic evaluation, peripheral tonometric measurements of peripheral PWV, central aortic blood pressure and augmentation index, cardiac magnetic resonance imaging (MRI) were analyzed at baseline and after 24 weeks of treatment.
Safety information was collected, including all adverse events and all serious adverse events. Completion of a serious adverse event form was required for all serious adverse events occurring during the study period. All serious adverse events were assessed by investigators and reported to the Novartis safety desk within 24hours.
The primary endpoint was central aortic distensibility by cardiac MRI at 24 weeks, reported as the change over the 24-week period after randomization. The secondary endpoints were central aortic PWV by cardiac MRI, change in central aortic blood pressure (hereafter, aortic blood pressure), augmentation index, peripheral PWV by tonometry, aortic root diameter by echocardiography, severity of aortic regurgitation by echocardiography, and dissection/rupture/operation of aneurysm.
Cardiovascular Imaging–echocardiography and Cardiac Magnetic Resonance ImagingCardiac Magnetic Resonance ImagingCardiac MRI was performed using a 1.5 Tesla scanner (Magnetom Avanto, Syngo MR; Siemens Medical Solutions, Erlangen, Germany). Aortic diameters were measured at 4 landmark levels: level 1, the ascending aorta at the level of bifurcation of the pulmonary artery; level 2, the upper descending thoracic aorta at the level of bifurcation of the pulmonary artery; level 3, the lower descending thoracic aorta at the level of the diaphragm; level 4, the abdominal aorta just above the iliac bifurcation. Cine imaging was also performed at the same levels to measure aortic stiffness.
Cardiac Magnetic Resonance Imaging Analysis–central Aortic Distensibility and Central Aortic Pulsed Wave VelocityAnalyses of MRIs were performed using commercial software (Argus version 4.02, Siemens Medical Systems, Germany) by experienced observers who were blinded to patient information. Central aortic distensibility and aortic PWV were measured according to a well-validated method using MRI.24,27 Distensibility = (Amax-Amin)/[Amin × (Pmax-Pmin)](10−3 mmHg−1), where Amax is the maximal (systolic) aortic area, Amin is the minimal (diastolic) aortic area, Pmax is the systolic blood pressure, and Pmin is the diastolic blood pressure. Central aortic blood pressure measured noninvasively by SphygmoCor was used for systolic and diastolic blood pressure. The PWV was measured at 2 regions: the proximal aorta (proximal PWV between level 1 and level 2) and the entire aorta (PWV-total between level 1 and level 4).
Measurement of Augmentation Index, Aortic Blood Pressure, and Peripheral Pulsed Wave VelocityPulse wave analysis was performed noninvasively with the SphygmoCor system (AtCor Medical, Sydney, Australia). Peripheral pressure waveforms were recorded from the radial artery at the wrist using applanation tonometry with a high-fidelity micromanometer. After acquisition of 20 sequential waveforms, a validated central aortic pressure waveform was generated by the mathematical formula using a fast Fourier transformation that resulted in a Food and Drug Administration-approved algorithm.28 The augmentation index and augmentation index adjusted by heart rate at 75 bpm were derived from the central aortic pressure.
The values of brachial-ankle PWV were simultaneously measured in each participant using a vascular testing device for applanation tonometry (VP-2000; Colin Medical Technology, Komaki, Japan).29
EchocardiographyAortic root measurements were made using the leading-to-leading edge method at end-diastole for up to 5 cycles and averaged. The Z-score represented the standard deviation from the diameter of the sinuses of Valsalva normalized for patient body surface area and age.30
Statistical AnalysisQuantitative variables are reported as mean ± standard deviation. The Mann-Whitney U test was used for continuous variables and the Fisher exact test was used for categorical variables when the 2 groups were compared. P-values < .05 were considered statistically significant. The change in central aortic distensibility from baseline to 24 weeks of treatment was compared between the negative control and aliskiren treatment groups9 using the generalized estimating equations approach to account for repeated measures and other possible confounding effects such as blood pressure, age, and sex. Other secondary endpoints were simply compared between the 2 groups using the Mann-Whitney U test.
Because the PWV is highly dependent on blood pressure levels, which decreased during the course of treatment, we adjusted the change in peripheral PWV at 24 weeks from baseline using a linear model that included changes in systolic blood pressure, diastolic blood pressure, sex, and age.
All analyses were performed using SPSS version 20.0 (SPSS Inc, Chicago, Illinois, United States) and R version 3.1.3.
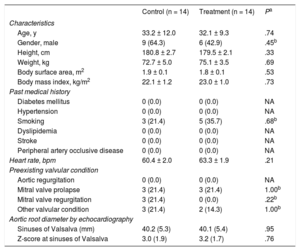
RESULTSBaseline Clinical CharacteristicsFrom November 2009 to October 2014, 28 patients (mean age 32.6 ± 10.6 years; 15 males [53.6%]) were enrolled and were randomly assigned to the aliskiren treatment group or the negative control group (n = 14 per group). All patients were receiving atenolol as standard beta-blocker therapy, and then aliskiren was added to the treatment group. The mean doses of atenolol were 47.3 ± 20.9mg in the aliskiren treatment group and 50.0 ± 19.6mg in the negative control group; P = .73. Alikiren was added in 14 patients in the treatment group and the mean dose was 289.3 ± 40.1mg.
Baseline variables are shown in Table 1. Clinical findings were similar in the aliskiren treatment group and negative control group. The mean aortic diameter (mm) at the level of the sinuses of Valsalva was 40.1 ± 5.4 vs 40.2 ± 5.3 (P = .95) at baseline with a mean Z-score of 3.2 ± 1.7 vs 3.0 ± 1.9 (P = .76) in the aliskiren treatment group vs control group. Pre-existing valvular conditions were not significantly different between groups.
The Baseline Clinical Characteristics of the Study Population
| Control (n = 14) | Treatment (n = 14) | Pa | |
|---|---|---|---|
| Characteristics | |||
| Age, y | 33.2 ± 12.0 | 32.1 ± 9.3 | .74 |
| Gender, male | 9 (64.3) | 6 (42.9) | .45b |
| Height, cm | 180.8 ± 2.7 | 179.5 ± 2.1 | .33 |
| Weight, kg | 72.7 ± 5.0 | 75.1 ± 3.5 | .69 |
| Body surface area, m2 | 1.9 ± 0.1 | 1.8 ± 0.1 | .53 |
| Body mass index, kg/m2 | 22.1 ± 1.2 | 23.0 ± 1.0 | .73 |
| Past medical history | |||
| Diabetes mellitus | 0 (0.0) | 0 (0.0) | NA |
| Hypertension | 0 (0.0) | 0 (0.0) | NA |
| Smoking | 3 (21.4) | 5 (35.7) | .68b |
| Dyslipidemia | 0 (0.0) | 0 (0.0) | NA |
| Stroke | 0 (0.0) | 0 (0.0) | NA |
| Peripheral artery occlusive disease | 0 (0.0) | 0 (0.0) | NA |
| Heart rate, bpm | 60.4 ± 2.0 | 63.3 ± 1.9 | .21 |
| Preexisting valvular condition | |||
| Aortic regurgitation | 0 (0.0) | 0 (0.0) | NA |
| Mitral valve prolapse | 3 (21.4) | 3 (21.4) | 1.00b |
| Mitral valve regurgitation | 3 (21.4) | 0 (0.0) | .22b |
| Other valvular condition | 3 (21.4) | 2 (14.3) | 1.00b |
| Aortic root diameter by echocardiography | |||
| Sinuses of Valsalva (mm) | 40.2 (5.3) | 40.1 (5.4) | .95 |
| Z-score at sinuses of Valsalva | 3.0 (1.9) | 3.2 (1.7) | .76 |
NA, not available.
Data are presented as mean ± standard deviation or No. (%).
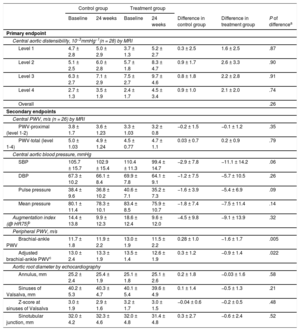
Central aortic distensibility (10−3 mmHg−1) and central PWV (m/s) by MRI at baseline and 24 weeks could be evaluated in 26 patients (Table 2). The MRI data of 2 patients could not be evaluated due to poor imaging quality. The change in central aortic distensibility from baseline to 24 weeks in the aliskiren treatment group indicated improvement at all respective levels (level 1, 1.6; level 2, 2.6; level 3, 2.2; level 4, 2.1 [10−3mmHg−1]), whereas values in the negative control group were only marginally improved (level 1, 0.3; level 2, 0.9; level 3, 0.8; level 4, 0.9 [10−3mmHg−1]). The differences in central distensibility at baseline vs 24 weeks showed a tendency for greater improvement in the aliskiren treatment group than in the negative control group. However, adjustment of confounding variables using generalized estimating equations indicated that the differences in distensibility were mainly related to systolic blood pressure, age, and sex, whereas the group difference was minimal and not statistically significant (P = .262 for all levels combined). Accordingly, the differences in central PWV by MRI at baseline vs 24 weeks were not significantly different between the aliskiren treatment group and negative control group.
Effect of Aliskiren on Arterial Stiffness Parameters, Blood Pressure, and Aortic Root Diametera
| Control group | Treatment group | ||||||
|---|---|---|---|---|---|---|---|
| Baseline | 24 weeks | Baseline | 24 weeks | Difference in control group | Difference in treatment group | P of differencea | |
| Primary endpoint | |||||||
| Central aortic distensibility, 10−3mmHg−1(n = 28) by MRI | |||||||
| Level 1 | 4.7 ± 2.8 | 5.0 ± 2.9 | 3.7 ± 1.3 | 5.2 ± 2.7 | 0.3 ± 2.5 | 1.6 ± 2.5 | .87 |
| Level 2 | 5.1 ± 2.5 | 6.0 ± 2.8 | 5.7 ± 1.8 | 8.3 ± 4.7 | 0.9 ± 1.7 | 2.6 ± 3.3 | .90 |
| Level 3 | 6.3 ± 2.7 | 7.1 ± 2.9 | 7.5 ± 2.7 | 9.7 ± 4.6 | 0.8 ± 1.8 | 2.2 ± 2.8 | .91 |
| Level 4 | 2.7 ± 1.3 | 3.5 ± 1.9 | 2.4 ± 1.7 | 4.5 ± 3.4 | 0.9 ± 1.0 | 2.1 ± 2.0 | .74 |
| Overall | .26 | ||||||
| Secondary endpoints | |||||||
| Central PWV, m/s (n = 26) by MRI | |||||||
| PWV-proximal (level 1-2) | 3.8 ± 1.7 | 3.6 ± 1.23 | 3.3 ± 1.03 | 3.2 ± 0.8 | −0.2 ± 1.5 | −0.1 ± 1.2 | .35 |
| PWV-total (level 1-4) | 5.0 ± 1.03 | 4.9 ± 1.24 | 4.5 ± 0.77 | 4.7 ± 1.1 | 0.03 ± 0.7 | 0.2 ± 0.9 | .79 |
| Central aortic blood pressure, mmHg | |||||||
| SBP | 105.7 ± 15.7 | 102.9 ± 15.4 | 110.4 ± 11.3 | 99.4 ± 14.7 | −2.9 ± 7.8 | −11.1 ± 14.2 | .06 |
| DBP | 67.3 ± 10.2 | 66.1 ± 8.4 | 69.9 ± 7.8 | 64.1 ± 9.1 | −1.2 ± 7.5 | −5.7 ± 10.5 | .26 |
| Pulse pressure | 38.4 ± 9.6 | 36.8 ± 10.2 | 40.6 ± 7.1 | 35.2 ± 7.3 | −1.6 ± 3.9 | −5.4 ± 6.9 | .09 |
| Mean pressure | 80.1 ± 11.4 | 78.3 ± 10.1 | 83.4 ± 8.5 | 75.9 ± 10.7 | −1.8 ± 7.4 | −7.5 ± 11.4 | .14 |
| Augmentation index (@ HR75)b | 14.4 ± 13.8 | 9.9 ± 12.3 | 18.6 ± 12.4 | 9.6 ± 12.0 | −4.5 ± 9.8 | −9.1 ± 13.9 | .32 |
| Peripheral PWV, m/s | |||||||
| Brachial-ankle PWV | 11.7 ± 1.8 | 11.9 ± 2.2 | 13.0 ± 1.9 | 11.5 ± 2.2 | 0.28 ± 1.0 | −1.6 ± 1.7 | .005 |
| Adjusted brachial-ankle PWVc | 13.0 ± 2.4 | 13.3 ± 1.9 | 13.5 ± 1.4 | 12.6 ± 1.9 | 0.3 ± 1.2 | −0.9 ± 1.4 | .022 |
| Aortic root diameter by echocardiography | |||||||
| Annulus, mm | 25.2 ± 2.4 | 25.4 ± 1.9 | 25.1 ± 1.8 | 25.1 ± 2.6 | 0.2 ± 1.8 | −0.03 ± 1.6 | .58 |
| Sinuses of Valsalva, mm | 40.2 ± 5.3 | 40.3 ± 4.7 | 40.1 ± 5.4 | 39.6 ± 4.9 | 0.1 ± 1.4 | −0.5 ± 1.3 | .21 |
| Z-score at sinuses of Valsalva | 3.0 ± 1.9 | 2.9 ± 1.6 | 3.2 ± 1.7 | 3.0 ± 1.5 | −0.04 ± 0.6 | −0.2 ± 0.5 | .48 |
| Sinotubular junction, mm | 32.0 ± 4.2 | 32.3 ± 4.6 | 32.0 ± 4.8 | 31.4 ± 4.8 | 0.3 ± 2.7 | −0.6 ± 2.4 | .52 |
DBP, diastolic blood pressure; MRI, magnetic resonance imaging; PWV, pulse wave velocity; SBP, systolic blood pressure.
Data are presented as mean ± standard deviation.
Comparison of changes in central aortic blood pressure measured by SphygmoCor from baseline to 24 weeks showed that systolic aortic blood pressure tended to be lower in patients in the aliskiren treatment group (−11.1mmHg) than in the control group (–2.9mmHg); however, this difference did not reach statistical significance (P = .09) (Table 2).
The difference from baseline to 24 weeks in peripheral PWV measured as brachial-ankle PWV (13.0 ± 1.9 m/s at baseline to 11.5 ± 1.8 m/s at 24 weeks in the aliskiren treatment group vs 11.7 ± 2.2 m/s to 11.9 ± 2.2 m/s in the negative control group; P = .005) showed a significant decrease in the aliskiren treatment group (Table 2). When a linear model was fit to peripheral PWV with regard to systolic blood pressure, diastolic blood pressure, and age, the following relationship explained 72% of the interpatient variance at baseline for all patients: peripheral PWV (m/s) = –3.11 + 0.087 systolic blood pressure (mmHg) + 0.065 diastolic blood pressure (mmHg) + 0.055 age (years). This means that the decrease can be explained primarily by a reduction in blood pressure with treatment, as the treatment has a known effect of lowering blood pressure. Therefore, we attempted to explain the decrease in peripheral PWV for these confounding effects by a linear model. The change in peripheral PWV at 24 weeks from baseline was significantly explained by differences in systolic blood pressure, age, and sex with an R-square of 82%. However, even after removing these influences, the difference between the 2 groups was approximately 1.2 m/s and highly significant with a P-value of .022 (Table 2).
Aortic Root Diameters by EchocardiographyAt the initial examination, all echocardiographic variables were comparable between the 2 groups (Table 2). The decreases in aortic root diameters of the annulus (P = .58), sinuses of Valsalva (P = .21), and sinotubular junction (P = .52) were not significantly attenuated in the aliskiren treatment group, and the difference in Z-score at the sinuses of Valsalva from baseline to 24 weeks was also not significantly different between groups (P = .48).
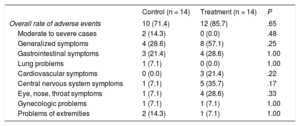
Adverse EventsThe number of patients with adverse events was higher in the aliskiren treatment group, but there was no significant difference between the aliskiren treatment group and the control group in the overall rate of adverse events (n = 12 [85.7%] vs n = 10 [71.4%]; Table 3). Regarding the occurrence of any adverse effects, most patients complained of mild symptoms. Moderate to severe adverse events occurred in 2 (14.3%) patients in the control group compared with no patients in the treatment group. These events consisted of 1 case of right inguinal hernia and 1 case of left pneumothorax, but both were considered not related to the administration of aliskiren.
All Adverse Events and Serious Adverse Events Between Patients in the Control and Treatment Groups
| Control (n = 14) | Treatment (n = 14) | P | |
|---|---|---|---|
| Overall rate of adverse events | 10 (71.4) | 12 (85.7) | .65 |
| Moderate to severe cases | 2 (14.3) | 0 (0.0) | .48 |
| Generalized symptoms | 4 (28.6) | 8 (57.1) | .25 |
| Gastrointestinal symptoms | 3 (21.4) | 4 (28.6) | 1.00 |
| Lung problems | 1 (7.1) | 0 (0.0) | 1.00 |
| Cardiovascular symptoms | 0 (0.0) | 3 (21.4) | .22 |
| Central nervous system symptoms | 1 (7.1) | 5 (35.7) | .17 |
| Eye, nose, throat symptoms | 1 (7.1) | 4 (28.6) | .33 |
| Gynecologic problems | 1 (7.1) | 1 (7.1) | 1.00 |
| Problems of extremities | 2 (14.3) | 1 (7.1) | 1.00 |
Data are expressed as No. (%).
The major finding of our study was that aliskiren therapy for 24 weeks did not reduce the central aortic stiffness relative to negative control therapy in patients with MS taking standard beta-blocker therapy. We did not observe the expected advantage of aliskiren therapy, although we did note a significant difference in favor of aliskiren with regard to the brachial-ankle PWV, which might be caused by lowered central aortic systolic pressure.
Up to 90% of MS patients will experience a cardiovascular event during their lifetime, including surgical repair of the aortic root, aortic dissection, or valve surgery.31 Aortic stiffness may be of additional prognostic value for aortic dilatation, dissection, and rupture in aortic degenerative disease and may contribute to risk stratification in patients at risk for aortic complications.32 Moreover, aortic stiffness is one of the earliest detectable signs of functional and structural changes in the vascular wall.17 Cardiac MRI represents an attractive modality for assessing central distensibility and central PWV because of its ability to visualize the actual aortic length and to identify regional stiffness.33 A previous study evaluated the diagnostic performance of regional PWV sampling with velocity-encoded MRI for the prediction of aortic luminal growth in MS patients. Magnetic resonance imaging-driven regional PWV assessment was reported to have moderate to high specificity for predicting the absence of regional aortic luminal growth for all aortic segments in MS patients.26 Therefore, aortic stiffness is a logical therapeutic target in adults with MS.
Even though aliskiren did not improve aortic stiffness as measured by central aortic distensibility or central PWV by MRI, peripheral PWV measured by brachial-ankle PWV was decreased following aliskiren treatment. The discrepancy between central and peripheral PWV could be explained by the lower central aortic systolic blood pressure in the aliskiren treatment group.
The pathogenesis of MS has recently been elucidated. Fibrillin-1 binds the latent complex of the cytokine TGF-β and regulates its activation and signaling.5,34 Studies in a mouse model of MS showed that fibrillin-1 deficiency was associated with excessive signaling by TGF-β. In an FBN1 mutation knock-in mouse model, losartan completely limited dilatation of the aortic root.5 It was suggested that excessive TGF-β signaling contributed to formation of the ascending aortic aneurysm, and that TGF-β antagonism with losartan represented a plausible treatment strategy. Cross-talk between the TGF-β system and the RAS has been demonstrated.34 The RAS blockers are developed at 3 levels: renin inhibitor, angiotensin-converting enzyme inhibitor, and ARB. The beneficial effects of RAS blockade by losartan and losartan add-on to beta-blocker therapy have been demonstrated in small-scale retrospective or prospective studies.7,35 In 233 adult MS patients, of whom more than 70% were taking a beta-blocker, losartan treatment reduced the aortic root dilatation rate and the dilatation rate of the aortic arch after aortic root replacement.8 However, larger-scale prospective studies question the applicability of the TGF-β hypothesis proposed in the mouse model to humans with MS. The Pediatric Heart Network Study failed to demonstrate superiority of losartan over atenolol in decreasing aortic dilatation in 608 children and young adults with MS.36 Very recently, the Marfan Sartan Trial did not show any benefit of losartan add-on to standardized beta-blocker therapy in 303 MS patients.10 Losartan compared with atenolol did not result in significant differences in the progression of aortic root and ascending aorta diameters in 140 MS patients.11
Compared with ARB and angiotensin-converting enzyme inhibitor, direct renin inhibitor, aliskiren has fewer adverse effects and may not lead to RAS “escape,” thus providing a greater RAS blockade.37,38 Aliskiren attenuates the expression or production of TGF-β. Aliskiren reduces TGF- β gene expression on cultured human aortic smooth muscle cells.12 In a nonhypertensive mouse model, aliskiren decreased the levels of TGF-β and accumulation of extracelluar matrix in the kidney.13 Another study has shown that the addition of aliskiren to valsartan exerts a synergistic protection against renal fibrosis through the attenuation of messenger RNA expression of TGF-β in a unilateral ureteral obstruction rat model.39 In a clinical study, aliskiren reduced the urinary excretion of TGF-β in patients with nondiabetic kidney disease.14 Furthermore, renin inhibitors behave as vasodilators with the potential to improve the elasticity of the large arteries.36,40 Transforming growth factor-beta suppression with aliskiren and its protective effect in aortic dilatation have not yet been demonstrated in MS patients. Our study could not show a beneficial effect of aliskiren with regard to aortic stiffness in MS patients treated with standard beta-blocker therapy.
LimitationsFirst, this study is limited by its small sample size. Second, a follow-up period of 6 months may have been relatively short to see improvements of aortic integrity and elasticity. Third, in a substudy of the COMPARE clinical trial, Franken et al., 41 reported that losartan reduces aortic diameter only in patients carrying FBN1 mutation of a haploinsufficiency. It is speculated that blockade of RAS acts only in patients with certain genotypes. Last, as differences in the losartan study design could have contributed to the controversial results regarding the aortic dilatation rate in the various studies, including study population, medication dosages, adherence to therapy, and imaging methods used,41 further studies are required to ascertain the effect of aliskiren therapy in certain subgroups regarding aortic root diameter, age, or type of gene mutation.
CONCLUSIONSIn conclusion, 24-week treatment with aliskiren with atenolol did not show a significant improvement in central aortic stiffness and diameter over atenolol alone. Long-term studies are required to ascertain the effect of aliskiren on structural changes of the aortic wall in patients with MS.
FUNDINGOur research grant was funded by Norvatis.
CONFLICTS OF INTERESTNone declared.
- –
Although recommended in clinical guidelines, there is limited evidence for the efficacy of beta-adrenergic blocker therapy in MS. After the effectiveness of losartan in a Marfan mouse model, losartan was speculated to be a more causative treatment through the interaction of the RAS and TGF-β system. However, recent large trials have shown discrepancies in the effect of losartan on aortic growth. Compared with losartan, the direct renin inhibitor, aliskiren, has fewer adverse effects and may not lead to RAS “escape”, thus providing a greater RAS blockade. To date, no study has analyzed the benefit of aliskiren in the treatment of MS.
- –
Aliskiren therapy for 24 weeks did not reduce central aortic stiffness relative to negative control therapy in patients with MS taking standard beta-blocker therapy. We did note a significant difference in favor of aliskiren with regard to the brachial-ankle PWV, which might be caused by lowered central aortic systolic pressure.
.