Keywords
INTRODUCTION
Contrast-induced nephropathy (CIN) is one of the most clinically important complications associated with the use of iodinated contrast media (CM). For nearly 2 decades, it has remained the third most common cause of acute renal failure in hospitalized patients1,2 with almost half of the cases occurring following coronary angiography and/or percutaneous coronary intervention (PCI).1 In the general population, the incidence of CIN is approximately 3% for patients who undergo coronary procedures3; however, in selected high-risk patient subsets, the risk for developing CIN can be as high as 50%.4-7
For patients who develop CIN following PCI, the prognostic impact is substantial.8 In addition to renal complications and higher systemic and cardiac complications, prolonged hospital stays and greater in-hospital mortality rates have been reported among patients who develop CIN as compared with those who do not.8 In addition, patients who are discharged from the hospital after developing CIN have significantly higher mortality rates as compared with those without CIN.3 Further, clinical studies have demonstrated a correlation between the magnitude of renal function change following coronary angiography and patient outcomes, suggesting that even small decreases in renal function following coronary angiography can be associated with increased mortality rates and prolonged hospital stays.9-11
Patients at highest risk for CIN include those with pre-existing renal impairment, particularly when it is secondary to diabetic nephropathy.12 Although diabetes per se, without reduced renal function, is not considered to be a risk factor for CIN,12 diabetic patients may have some degree of reduced renal function despite having normal serum creatinine (SCr) levels.13-15 A number of randomized, controlled clinical trials have demonstrated that use of iso-osmolar CM (IOCM) reduces the risk for CIN in patients with chronic renal impairment with or without diabetes who are undergoing coronary procedures.16-18 However, the benefit of IOCM in a population of diabetic patients has not been established.
The present study was designed to assess the incidence of CIN and SCr changes after administration of the IOCM iodixanol or the lowosmolar CM (LOCM) ioversol in a population of diabetic patients undergoing coronary angiography with or without PCI.
METHODS
Study Patients
This single-center, prospective, open-label study was conducted at Hospital Universitario 12 de Octubre in Madrid, Spain, between May 2005 and February 2007. Patients referred for coronary angiography with or without PCI were considered to be eligible if they had a history of diabetes and were being treated with insulin and/ or oral hypoglycemic agents. Exclusion criteria included: any emergency procedure (eg, primary angioplasty) that did not allow for adequate patient hydration; cardiogenic shock; previous heart or kidney transplantation or current use of immunosuppressive agents; renal disease requiring dialysis; administration of CM within the previous 7 days; lack of baseline or 72-hour postprocedure SCr measurement. The study protocol was reviewed and approved by the local ethics committee, and all patients gave informed consent before entry in the study.
Study Protocol
Coronary angiography and interventions were performed according to standard protocols for our institution using the radial or femoral approach. Inpatients were electively scheduled the day before the procedure and outpatients were admitted the same day and discharged home during the afternoon (if the procedure was a diagnostic one) or the following day (if a PCI was performed). The choice of contrast agent was governed by contractual arrangements such that the CM was changed systematically from ioversol (Optiray 350, Tyco Healthcare, Spain; 702 mOsm/kg water [May 2005 to November 2005]) to iodixanol (Visipaque 270, GE Healthcare, Buckinghamshire, UK; 290 mOsm/kg water [April 2006 to February 2007]). As such, patients enrolled during the first 7 months of the study received ioversol and patients enrolled during the following 11 months received iodixanol. Prophylactic volume expansion with 1000 mL intravenous normal saline was administered for 6 to 12 hours before the procedure (100 to 150 mL/h) and an oral dose of 1200 mg N-acetylcysteine (NAC) (Fluimucil®, Zambon, Milan, Italy) was administered 6 hours before and 6 hours after the procedure. Blood samples for SCr were obtained upon admission (at baseline) and at 72 hours postprocedure. Outpatients were scheduled to come back to the hospital at 72 hours for collection of blood samples. All SCr levels were measured in a central laboratory at Hospital Universitario 12 de Octubre. Estimated glomerular filtration rate (eGFR), as a measure of renal function, was calculated according to the Modification of Diet in Renal Disease (MDRD) study equation.19
Study Endpoints
The primary endpoint of this study was the incidence of CIN, defined as an absolute increase in SCr from baseline of >0.5 mg/dL or a relative increase of >25% at 72 hours following exposure to CM. The mean increase in SCr at 72 hours post-CM and independent predictors of CIN were secondary study endpoints. Based upon previous studies, a sample size of at least 100 patients per group was calculated and a higher number was included to allow for the possibility of patient exclusion or incomplete collection of data.
Statistical Analysis
Continuous variables were summarized by means of descriptive statistics. Categorical variables were expressed as percentages. Comparison between iodixanol and ioversol for the occurrence of CIN was tested with the 2-sided c2 test (categorical variables) and the Student t test (continuous variables). Mean changes in SCr between baseline and 72 hours post-CM were determined using analysis of variance (ANOVA). A multivariate logistic regression model was used to identify variables independently predictive of CIN. Potentially relevant variables included type of contrast, baseline eGFR, CM volume, age, sex, inpatient or outpatient status, insulin or oral hypoglycemic treatment, and PCI. All P values less than .05 were considered to be significant. Statistical analyses were performed using SPSS software version 14.0 (SPSS Inc., Chicago, Illinois).
RESULTS
Patient Demographics and Disposition
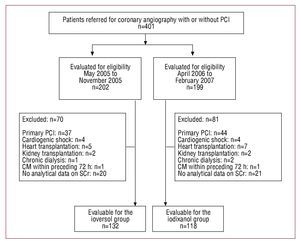
A total of 401 consecutive patients referred for coronary angiography or PCI were assessed for eligibility. This included 202 patients who received ioversol (May to November, 2005), as well as 199 patients who received iodixanol after it became available for use in April 2006. Among these, 151 failed to meet the inclusion criteria (Figure). In totals 250 patients were enrolled in the study: 132 received ioversol and 118 received iodixanol. The demographic, clinical, and procedural characteristics of the patients are shown in Table 1. The 2 treatment groups were similar with regard to mean age, gender distribution, baseline SCr levels, and baseline eGFR. All study patients had type 2 diabetes, except for 1 patient in the ioversol group, who had type 1 diabetes. Medications administered before catheterization were not discontinued. Although use of angiotensin-converting enzyme inhibitors, diuretics, and statins was similar between groups, significantly more patients in the iodixanol group received insulin (P=.019) while more patients in the ioversol group received oral hypoglycemics (P<.0005).
Figure 1. Flow chart corresponding to the patients in the study. CM indicates contrast medium; PCI, percutaneous coronary intervention; SCr, serum creatinine concentration.
Incidence of Contrast-Induced Nephropathy
The incidence of CIN in the 2 study groups is presented in Table 2. The overall incidence of CIN was 5.6%. An absolute increase greater than 0.5 mg/dL or a relative increase of more than 25% in SCr within 72 hours of contrast was observed in 2.5% (3 of 118) of the patients in the iodixanol group and in 8.3% (11 of 132) of the patients in the ioversol group (P=.047). In the subset of patients who underwent PCI, the incidence of CIN was significantly lower among those who received iodixanol than among the patients who received ioversol (P=.031). Significantly fewer hospital inpatients who received iodixanol developed CIN as compared with those who received ioversol (P=.023). The incidence of CIN among patients with eGFR less than or equal to 60 mL/min/1.73m2 was lower for patients receiving iodixanol (2 of 41; 4.9%) compared with that for patients who received ioversol (6 of 35; 17.1%), but only showed a trend that did not reach statistical significance (P=.082).
Mean Increase in Serum Creatinine
The mean increases in SCr levels from baseline to 72 hours post-CM are seen in Table 3. For patients receiving ioversol, the mean SCr increased significantly from a baseline of 1.06 (0.46) mg/ dL to a peak of 1.13 (0.60) mg/dL (P=.008; 95% confidence interval [CI], 0.018-0.114 mg/dL). By comparison, among patients who received iodixanol, the mean change in SCr was not significant, increasing from 1.04 (0.43) mg/dL at baseline to 1.06 (0.45) mg/dL at 72 hours post-CM (P=.454; 95% CI, -0.032 to 0.070 mg/dL). The mean increase in SCr was also significantly greater among patients with a baseline eGFR lower than or equal to 60 mL/min/1.73m2 who received ioversol (P=.038), whereas among those patients who received iodixanol, the mean change in SCr was not significant (P=.533). Among patients who underwent PCI and received ioversol, the mean increase in SCr was significant (P=.011). No significant mean change in SCr was found among patients in the iodixanol group who underwent PCI (P=.336). In this study, no patient with CIN required dialysis.
Risk Factors for Contrast-Induced Nephropathy
Multivariate logistic regression analysis identified the type of CM and the baseline eGFR as independent predictors of CIN in this patient population. The use of iodixanol was found to be protective compared to the use of ioversol (odds ratio [OR], 0.255; 95% CI, 0.068-0.952). Low eGFR, 60.8 (29) mL/min/1.73 m2 (in patients who developed CIN) versus 75.3 (25) mL/min/1.73 m2 (in patients who did not develop CIN) (OR, 0.975; 95% CI, 0.952-0.997; P=.03) was also an independent predictor of CIN. There was no significant relationship between occurrence of CIN and CM volume or intervention.
DISCUSSION
Our results show that the use of iodixanol resulted in a significantly lower incidence of CIN than the use of ioversol in diabetic patients undergoing coronary angiography with or without PCI. When CIN is defined as an increase in SCr from a baseline of more than 25% or 0.5 mg/dL within 72 hours of CM administration, 2.5% of the patients in the iodixanol group developed CIN compared with 8.3% of the patients in the ioversol group (P=.047). The use of iodixanol compared with ioversol also resulted in a significantly lower rate of CIN in the subgroup of patients who underwent PCI, as well as among hospital inpatients. These findings are relevant because the present study was conducted in a cohort of diabetic patients, 70% of whom had normal baseline eGFR levels, a population in whom prophylactic CIN-preventive strategies may not be routinely implemented in clinical practice. However, even though the diabetic patients in the present study did undergo prophylactic volume expansion prior to CM administration, those who received ioversol had a significantly greater incidence of CIN compared with those who received iodixanol.
There is a growing body of evidence suggesting that CM osmolality is an important factor in the development of CIN in patients with renal impairment with or without diabetes.5,20,21 Initial studies demonstrated that LOCM were associated with a lower incidence of CIN as compared with high-osmolar CM when used in patients at risk of renal injury.5,20 More recent clinical trials in the cardiology setting have shown that use of the IOCM iodixanol significantly reduces the incidence of CIN when compared with the LOCM iohexol, ioxaglate and iopromide in at-risk patients.16-18 The Nephrotoxicity in High-Risk Patients Study of Iso-Osmolar and Low-Osmolar Non-Ionic Contrast Media (NEPHRIC) trial was a randomized, multicenter, double-blind trial comparing the nephrotoxicity of iodixanol and iohexol in diabetic patients with SCr levels between 1.5 and 3.5 mg/dL16 who were undergoing coronary or aortofemoral angiography. The incidence of CIN, defined as an increase ≥0.5 mg/dL in SCr between day 0 and day 3, was 3% in the iodixanol group and 26% among the patients who received iohexol (P=.002). A similar benefit of iodixanol was found in the Renal Toxicity Evaluation and Comparison Between Visipaque and Hexabrix in Patients With Renal Impairment Undergoing Coronary Angiography (RECOVER) trial.17 When defined as an increase in SCr ≥25% or ≥0.5 mg/dL within 2 days of exposure to the CM, the incidence of CIN was 7.9% among patients who received iodixanol and 17.0% among those who received ioxaglate (P=.021). The incidence of CIN was also significantly lower for the subset of patients with renal impairment and diabetes who received iodixanol compared with those who received ioxaglate (P=.041). Most recently, the 2007 American College of Cardiology/ American Heart Association guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction recommended the use of iso-osmolar CM in patients with chronic kidney disease (CKD) who undergo angiography.22
One recent randomized trial that included patients with stable kidney disease (eGFR, 20-59 mL/min/1.73 m2) who underwent cardiac catheterization procedures failed to show a difference in the rate of CIN between the IOCM iodixanol and the LOCM iopamidol.23 In this study by Solomon and coworkers, the postdose increase in SCr ≥0.5 mg/dL from baseline was not significantly different between the two study groups for all patients (4.4% with iopamidol vs 6.7% with iodixanol; P=.39). Although other randomized controlled trials in the coronary angiography setting used fixed time points to measure postprocedure SCr levels,16-18 Solomon and coworkers23 employed a single, random SCr measurement at any time between 45 and 120 hours postprocedure. Because different CM may exert their maximal effect on the kidney at different times in different individuals,5,24 the use of nonstandardized measurements of SCr at random time points may not accurately reflect the true incidence of CIN in a given population. In fact, depending on the time the sample was extracted, there was a higher incidence of CIN with one or the other CM.23
Although it is widely accepted that patients at greatest risk for CIN are those with both renal impairment and diabetes,12 the literature has been inconsistent as to whether diabetics without renal impairment are at increased risk for CIN.6,12,25 Recently, a number of studies have demonstrated that, despite normal SCr levels, CKD is prevalent among diabetics.13-15 Current screening techniques, including SCr, fail to identify a considerable number of diabetics with moderate to severe CKD13 and simple tools for detecting trends in early renal function decline among diabetics are lacking.26 The results of one observational study conducted at a hospital in the United Kingdom demonstrated that 60.6% of more than 7500 diabetic patients had stage 3 CKD (GFR, 30-59 mL/min/1.73 m2) with normal SCr levels.13 A high prevalence of stage 2 (GFR, 60-89 mL/min/1.73 m2) and stage 3 CKD was also found in diabetic patients with normal SCr levels undergoing PCI.14 Bachorzewska-Gajewska et al demonstrated that up to 77% of nearly 300 diabetic patients with normal SCr had CKD and suggested that the risk for CIN is enhanced among diabetic patients with normal SCr levels who undergo PCI.14 Similarly, in a recent study of patients with non-ST elevation acute coronary syndrome and normal plasma creatinine levels (SCr=1.3 mg/dL) who underwent coronary angiography, 22% had a CrCl <60 mL/min.27 Although the baseline SCr level did not correlate with the development of CIN, patients who developed CIN had lower baseline CrCl levels than patients who did not develop CIN (P<.001).27 The results of our study support the findings that, despite normal SCr, diabetics can have some degree of reduced renal function, and suggest that, in a real-world setting, iodixanol offers greater renal protection than ioversol in these patients when they undergo coronary angiography with or without PCI.
At this time, there are relatively few published data on the incidence of CIN in diabetic patients who have normal SCr. We are aware of one other report, by Hardiek and coworkers, in which iodixanol and iopamidol were compared in diabetic patients with normal or mild renal dysfunction (SCr=2 mg/dL) undergoing diagnostic or interventional coronary angiography.28 In their randomized trial, blood samples for SCr determination were obtained at baseline, before intravenous hydration, and on days 1, 3, and 7 postprocedure. Throughout the entire study period, 21% of the patients who received iopamidol had an SCr increase ≥25% compared with 13% of those treated with iodixanol (not significant). A similar nonsignificant difference was noted over the study period when CIN was defined as an SCr increase ≥0.5 mg/dL. On day 7, none of the iodixanol-treated patients had an SCr increase ≥0.5 mg/dL, compared with 8% of those treated with iopamidol (P=.045). Although the authors concluded that the renal effects of iopamidol and iodixanol were comparable in their "moderate risk" population, the true level of risk of their patient population is unclear.6,10 In their study, baseline CrCl, derived using the Cockcroft-Gault equation, was 105.5 (44.5) mL/min in the iodixanol group and 117.1 (57.9) mL/min in the iopamidol group. On the basis of the National Kidney Foundation Guidelines, these values are in the normal range.29 By comparison, in the present study, baseline eGFR was 72.7 (26.4) mL/min/1.73 m2 among iodixanol-treated patients and 76.1 (25.1) mL/min/1.73 m2 among those who received ioversol, indicating that patients had stage 2 CKD. Thus, differences in baseline renal function between the patients in our study and the "healthier" patients in the Hardiek study may account for the difference in the results.
The use of ioversol and low baseline eGFR were identified as independent risk factors for CIN in the present study. As compared with the use of ioversol, iodixanol was found to be protective against the development of CIN. With other factors remaining constant, for every 100 ioversol-treated patients developing CIN, 25.5 iodixanol-treated patients would develop CIN (risk reduction of 75%). Our findings are consistent with previous reports that identified baseline eGFR <60 mL/ min/1.73 m2 and CKD as a risk factor for CIN in patients undergoing PCI.7,30 We demonstrated that, for every 1 mL/min/1.73m2 increase in eGFR, the risk of developing CIN is reduced by 2.5%.
We recognize a number of limitations to this study: it is a single-center design with a limited sample size (though similar to many of the studies in the literature), but the main limitation is the nonrandomized sequential design of the study. On the other hand, this study was undertaken to evaluate "real-world" practice rather than very carefully controlled conditions, such as those of a randomized clinical trial.
In addition, we calculated GFR using the Modification of Diet in Renal Disease study equation. Although this formula has been widely used as a measure of renal function in CIN studies, it has not been validated in a large cohort of individuals with diabetes.31 Other markers of renal function, such as cystatin C, may provide a more accurate estimate of renal function in patients with diabetes.6,31,32 Finally, while we used a fixed time point (72 hours) to measure post-CM SCr, two or more fixed measurements may better reflect the development of CIN. Clearly, larger, well-designed, randomized, controlled trials are needed to confirm our findings.
CONCLUSIONS
In conclusion, iodixanol was associated with a significantly lower incidence of CIN than ioversol when used in diabetic patients undergoing coronary angiography with or without PCI. Given the current epidemic of diabetes and the complications associated with this disease, increasing numbers of patients with diabetes are likely to undergo contrast-enhanced imaging procedures in the future. Our results add to the growing body of evidence showing a benefit of iso-osmolar contrast agents in patients undergoing coronary procedures.
ACKNOWLEDGMENTS
We greatly appreciate the statistical support of Jesús Garrido, PhD.
ABBREVIATIONS
CIN: contrast-induced nephropathy
CKD: chronic kidney disease
CM: contrast medium
eGFR: estimated glomerular filtration rate
PCI: percutaneous coronary intervention
SCr: serum creatinine
Correspondence: Dr. F. Hernández.
Unidad de Hemodinámica y Cardiología Intervencionista. Hospital Universitario 12 de Octubre.
Av Córdoba s/n. 28041 Madrid. España.
E-mail: fhernandezh@medynet.com
Received January 13, 2009.
Accepted for publication September 16, 2009.